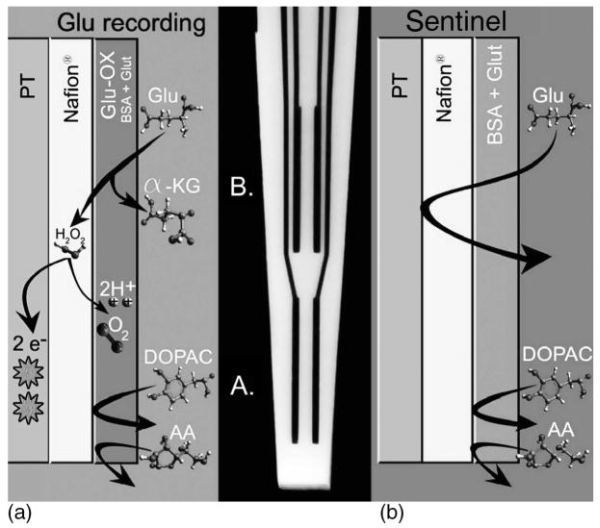
FIGURE 19.10
Schematic and photomicrograph of an S2, Nafion coated, self-referencing, L-glutamate selective multisite microelectrode. (a) Schematic showing the bottom pair of Pt recording sites coated with L-glutamate oxidase (Glu-ox), BSA, and glutaraldehyde (Glut). L-glutamate that comes into contact with the Gluox is converted to α-ketoglutarate and peroxide. The peroxide readily diffuses through the Nafion layer that blocks anionic substances such as AA and DOPAC. When the peroxide formed comes into contact with the Pt recording sites at a potential of + 0.7 V versus a Ag/AgCl reference electrode, the peroxide is oxidized and the resulting two electrons are transferred to the Pt recording site and the change in current is measured by the FAST-16 system. (b) Schematic showing the top pair of Pt recording sites coated with the inactive protein matrix of BSA and Glut, also referred to as the sentinel sites. Since no Glu-ox is coated onto this pair of recording sites, L-glutamate is not converted to a reporter molecule and thus is not measured on the Pt recording sites. Once again, the Nafion on these sites repels AA and DOPAC.
- Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS.[J Neurosci Methods. 2002]Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS.Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. J Neurosci Methods. 2002 Sep 30; 119(2):163-71.
- Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.[In Vivo Optical Imaging of Bra...]Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.Rector DM, Yao X, Harper RM, George JS. In Vivo Optical Imaging of Brain Function. 2009
- Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain.[J Neurochem. 2006]Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain.Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. J Neurochem. 2006 Mar; 96(6):1626-35. Epub 2006 Jan 25.
- Simultaneous glutamate recordings in the frontal cortex network with multisite biomorphic microelectrodes: New tools for ADHD research.[J Neurosci Methods. 2015]Simultaneous glutamate recordings in the frontal cortex network with multisite biomorphic microelectrodes: New tools for ADHD research.Miller EM, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA, Glaser PE. J Neurosci Methods. 2015 Aug 30; 252:75-9. Epub 2015 Jan 19.
- Review Electrochemical techniques for subsecond neurotransmitter detection in live rodents.[Comp Med. 2014]Review Electrochemical techniques for subsecond neurotransmitter detection in live rodents.Hascup KN, Hascup ER. Comp Med. 2014 Aug; 64(4):249-55.
- FIGURE 19.10, [Schematic and photomicrograph of an...]. - Electrochemical Method...FIGURE 19.10, [Schematic and photomicrograph of an...]. - Electrochemical Methods for Neuroscience
Your browsing activity is empty.
Activity recording is turned off.
See more...
