By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.

Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.
Show detailsMyelin in situ has a water content of about 40%. The dry mass of both CNS and PNS myelin is characterized by a high proportion of lipid (70 to 85%) and, consequently, a low proportion of protein (15 to 30%). In contrast, most biological membranes have a higher ratio of proteins to lipids.
Central nervous system myelin is enriched in certain lipids
Table 4-1 lists the composition of bovine, rat and human myelin compared to bovine and human white matter, human gray matter and rat whole brain (see Chap. 3). All of the lipids assayed in whole brain are also present in myelin; that is, there are no lipids localized exclusively in some nonmyelin compartment, with the exception of the mitochondria-specific lipid diphosphatidylglycerol, not included in the table. We also know that the reverse is true; that is, there are no myelin lipids that are not also found in other subcellular fractions of the brain.
Table 4-1
Composition of CNS Myelin and Brain.
Even though there are no absolutely “myelin-specific” lipids, cerebroside, also known as galactosylceramide, is the most typical of myelin. With the exception of early development, the concentration of cerebroside in brain is directly proportional to the amount of myelin present. As much as one-fifth of the total galactolipid in myelin occurs in the form of sulfatide, in which the 3-hydroxyl moiety on the galactose of cerebroside is sulfated. Because of the specificity and quantitative significance of galactocerebroside in oligodendrocytes and myelin, it has been assumed for decades that it is essential for oligodendroglial differentiation and the specialized structure and function of myelin. This dogma was overthrown by the creation of a knockout mouse lesioned in UDP-galactose:ceramide galactosyltransferase, the obligate terminal step in cerebroside biosynthesis, also required for sulfatide formation [10]. Surprisingly, the myelin formed appears relatively normal, although subtle differences in structure and changes in axon conduction velocity can be demonstrated. With age, animals develop a progressive hindlimb paralysis and extensive vacuolation of myelin in the spinal cord. These findings indicate that cerebroside and/or sulfatide are not required for myelin formation but play important roles in its insulating capacity and stability.
In addition to cerebroside, the major lipids of myelin are cholesterol and ethanolamine-containing plasmalogens (see Chap. 3). Lecithin is also a major myelin constituent, and sphingomyelin is a relatively minor one. Not only is the lipid composition of myelin highly characteristic of this membrane, the fatty acid composition of many of the individual lipids is distinctive.
The data in Table 4-1 suggest that myelin accounts for much of the total lipid of white matter and that the lipid composition of gray matter is quite different from that of myelin. The composition of brain myelin from all mammalian species studied is very much the same. There are, however, some species differences; for example, myelin of rat has less sphingomyelin than bovine or human myelin (Table 4-1). Although not shown in the table, there are also regional variations; for example, myelin isolated from the spinal cord has a higher lipid-to-protein ratio than brain myelin from the same species.
Besides the lipids listed in Table 4-1, there are several others of importance. If myelin is not extracted with acidified organic solvents, the polyphosphoinositides (see Chap. 3) remain tightly bound to the myelin protein and, therefore, are not included in the lipid analysis. Triphosphoinositide accounts for between 4 and 6% of the total myelin phosphorus and diphosphoinositide for 1 to 1.5%.
Minor components of myelin include at least three fatty acid esters of cerebroside and two glycerol-based lipids, diacylglyceryl-galactoside and monoalkylmonoacylglycerylgalactoside, collectively called galactosyldiglyceride. Some long chain alkanes also appear to be present. Myelin from mammals contains 0.1 to 0.3% gangliosides, which are complex sialic acid-containing glycosphingolipids. The proportion of the different gangliosides to each other is different in myelin, which is greatly enriched in monosialoganglioside GM1 relative to other brain membranes, which are enriched in the polysialo species. Myelin from certain species, including human, contains an additional unique ganglioside as a major component, sialosylgalactosylceramide, GM4.
Peripheral and central nervous system myelin lipids are qualitatively similar
There are quantitative differences. PNS myelin has less cerebroside and sulfatide and considerably more sphingomyelin than CNS myelin. Of interest is the presence of ganglioside LM1, also termed sialosyl-lactoneotetraosylceramide, as a characteristic component of myelin in the PNS of some species. These differences in lipid composition between CNS and PNS myelin are not, however, as dramatic as the differences in protein composition discussed below.
Central nervous system myelin contains some unique proteins
The protein composition of CNS myelin is simpler than that of other brain membranes, with the proteolipid protein and basic protein(s) making up 60 to 80% of the total in most species. Many other proteins and glycoproteins are present to lesser extents. With the exception of the basic proteins, myelin proteins are neither easily extractable nor soluble in aqueous media. However, like other membrane proteins, they may be solubilized in sodium dodecylsulfate (SDS) solutions and, in this condition, separated readily by electrophoresis in polyacrylamide gels. This technique separates proteins primarily according to molecular weight. The presence of bound carbohydrates or unusual structural features somewhat disrupts the relationship between migration and molecular weight so that terminology for location of a protein in such a gel is taken to mean apparent molecular weight, which sometimes is written Mr for relative molecular mass. Protein compositions of human and rat brain myelin are illustrated in Figure 4-11B and D, respectively. The quantitative predominance of two proteins, the positively charged myelin basic protein (MBP) and proteolipid protein (PLP), in the gel pattern of human CNS myelin is clear. These proteins are major constituents of all mammalian CNS myelins, and similar proteins are present in myelins of many lower species.
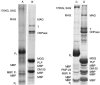
Figure 4-11
Polyacrylamide gel electrophoresis of myelin proteins in the presence of sodium dodecyl sulfate (SDS). The proteins of A: human PNS myelin, B: human CNS myelin, C: rat PNS myelin and D: rat CNS myelin were solubilized with the detergent SDS, electrophoresed (more...)
Proteolipid protein. Myelin PLP, also known as the Folch-Lees protein [11], has the unusual physical property of solubility in organic solvents. The molecular weight of PLP from sequence analysis is about 30,000, although it migrates anomalously fast on SDS gels. The amino acid sequence, strongly conserved during evolution, contains several membrane-spanning domains. PLP contains about 3 moles of fatty acids, primarily palmitate, oleate or stearate, per mole protein in ester linkage to hydroxy amino acids. There is rapid turnover of the fatty acids independent of the peptide backbone [12].
In addition to PLP, myelin of the CNS has lesser quantities of a related protein, DM-20, named for its Mr of 20,000. This protein is coded by an alternative splicing of the RNA, which gives rise to the major PLP. Both DNA and protein-sequencing data indicate that the structure of DM-20 is related to that of PLP by a deletion of 35 amino acids [13,14]. DM-20-related message appears earlier than PLP during development, even before myelin formation in some cases; and it might have a role in oligodendrocyte differentiation in addition to a structural role in myelin. The PLP and DM-20 proteins may be evolved from an ancestral gene encoding a pore-forming polypeptide [15], lending support to the hypothesis that myelin may be involved in ion movement. Although PLP and DM-20 serve important functions, they are not essential. Contrary to the general expectation that PLP would be needed for formation of compact, multilamellar myelin, a knockout mouse for PLP/DM-20 [16] is relatively normal with respect to myelin formation, although there is a difference at the level of the intraperiod line. In this knockout mouse, life span and sophisticated motor performance also are affected. In contrast, a variety of naturally occurring mutations in PLP (see Chap. 39) or overexpression of normal PLP [17] have severe functional consequences, apparently due to cellular toxicity of mutated forms of the protein or even just excess amounts of normal PLP. A curiosity is that, although significant amounts of PLP and DM-20 are restricted to the CNS, mRNA for PLP is expressed in the PNS and small amounts of protein are synthesized but not incorporated into myelin in appreciable amounts.
Myelin basic protein has long been of interest because it is the antigen that, when injected into an animal, elicits a cellular immune response that produces the CNS autoimmune disease experimental allergic encephalomyelitis (EAE) (see Chap. 39). MBP can be extracted from myelin as well as from white matter with either dilute acid or salt solutions; once extracted, it is very soluble in water. The amino acid sequence of the major basic protein is similar in a number of species [11]. These proteins have molecular weights of around 18,500; they are highly unfolded, with essentially no tertiary structure in solution. This basic protein shows microheterogeneity upon electrophoresis in alkaline conditions, due to a combination of phosphorylation, loss of the C-terminal arginine and deamidation. There is also heterogeneity in the degree of methylation of an arginine at residue 106. MBP is located on the cytoplasmic face of the myelin membranes corresponding to the major dense line. The rapid turnover of the phosphate groups present on many of the MBP molecules [18,19] suggests this post-translational modification might influence the close apposition of the cytoplasmic faces of the membrane. It also has been speculated that phosphorylation may modify this process in a dynamic manner. Of interest is that mRNA coding for MBP is preferentially localized far from the cell perikaryon, in the region where myelin compaction is taking place [20].
In addition to the major MBP, most species of mammals that have been studied contain various amounts of other basic proteins related to it in sequence. Mice and rats have a second smaller MBP of 14-kDa. The small MBP has the same N- and C-terminal sequences as the larger MBP but differs by a deletion of 40 residues. The ratio of these two basic proteins to each other changes during development: mature rats and mice have more of the 14-kDa protein than of the 18-kDa protein. Two other MBPs seen in many species have molecular weights of 21,500 and 17,000, respectively. These two proteins are related structurally to the large and small basic proteins, respectively, by the addition of a polypeptide sequence of Mr ~3,000 near the amino-terminal end of the protein. Another basic protein, present to some extent in humans, has a molecular weight of 17,200 and is now known to be slightly different from the 17-kDa protein in other species. The different MBPs arise from alternative splicing of a common mRNA precursor. A diagrammatic representation of some of these alternative splicing schemes is presented in Figure 4-12. The physiological significance of the heterogeneity of MBPs is an open question. It is relevant that the exons which can be combined to make the various myelin basic proteins are also part of a larger set of exons of a gene, GOLLI. Transcripts of this gene are expressed as Golli proteins, which contain MBP sequences as well as unique peptide sequences, during early development and in various neural cell types, including neurons [22].
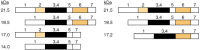
Figure 4-12
The amino acid sequences corresponding to the various mouse myelin basic proteins (MBPs) are encoded in a gene containing at least seven exons (separated by introns, DNA regions whose base sequence does not code directly for proteins). The precursor RNA (more...)
2′:3′-Cyclic nucleotide-3′-phosphodiesterase. There are many higher molecular weight proteins present in the gel-electrophoretic pattern of myelin. These vary in amount depending on species; for example, mouse and rat may have as much as 30% of the total myelin protein in this category. These proteins also vary depending on the degree of maturity, such that the younger the animal, the less myelin but the greater the proportion of higher molecular weight proteins. A double band with Mr ~50,000 is present in myelin from most species. It has been identified with an enzyme activity, 2′:3′-cyclic nucleotide-3′-phosphodiesterase (CNP), which comprises several percent of myelin protein [23]. Although there are low levels of this enzymatic activity associated with the surface membrane of many different types of cell, it is much enriched in myelin and in cells committed to the formation of myelin. The enzyme is extremely active against 2′,3′-cAMP, as well as the cGMP, cyclic cytidine monophosphate (cCMP) and cyclic uridine monophosphate (cUMP) analogs, all of which are hydrolyzed to the corresponding 2′-isomer. This is probably an artifactual activity; recall that the biologically active cyclic nucleotides are those with a 3′:5′ structure. The amino acid sequence is relatively conserved in different species. In mice, the two CNP polypeptides are generated by alternative splicing of the mRNA, with the larger polypeptide having an extra 20 amino acids at the N-terminus.
CNP is not a major component of compact myelin but is concentrated in specific regions of the myelin sheaths associated with cytoplasm, such as the oligodendroglial processes, inner and outer tongue processes and lateral loops. The biological function of CNP is not known, but it is of interest that it contains a consensus sequence found in G proteins. It also has been proposed, in part because of its isoprenylation, that CNP plays a role in events involving the cytoskeletal network of myelin [24]. Examination of aberrant myelination occurring in transgenic mice overexpressing CNP suggests that it is an early regulator of cellular events that culminate in CNS myelination [25].
Myelin-associated glycoprotein and other glycoproteins of CNS myelin. The myelin-associated glycoprotein (MAG) is a quantitatively minor, 100-kDa glycoprotein in purified myelin [26] that electrophoreses at the position shown in Figure 4-11. However, because it is less than 1% of total protein and stains weakly with Coomassie blue, it does not correspond to one of the discrete protein bands visible in the figure. MAG has a single transmembrane domain that separates a heavily glycosylated extracellular part of the molecule, composed of five immunoglobulin-like domains and eight or nine sites for N-linked glycosylation, from an intracellular carboxy-terminal domain. Its overall structure is similar to that of neural cell adhesion molecule (NCAM). MAG in rodents occurs in two developmentally regulated isoforms, which differ in their cytoplasmic domains and are generated by alternative splicing of mRNA. The isoform with a longer C-terminal tail (L-MAG) is predominant early in development during active myelination of the CNS, whereas the isoform with a shorter cytoplasmic tail (S-MAG) increases during development to become prominent in adult rodents. These cytoplasmic domains are phosphorylated on serine and threonine residues by protein kinase C, and L-MAG is phosphorylated also on tyrosine-620.
MAG is not present in compact, multi-lamellar myelin but is located exclusively in the periaxonal oligodendroglial membranes of CNS myelin sheaths. Both its location next to the axon and its membership in the immunoglobulin superfamily (see Chap. 7) suggest that it functions in adhesion and signaling between myelin-forming oligodendrocytes and the axolemma. MAG is a member of the I-type lectin subfamily of the immunoglobulin superfamily and binds to glycoproteins and gangliosides with terminal 2–3-linked sialic acid moieties. Thus, the axolemmal ligand(s) for MAG is probably a sialoglycoconjugate, but the identity of its physiological receptor is unknown. Furthermore, MAG may bind to other components on the axolemma by different mechanisms. A relationship of MAG to other adhesion proteins also is demonstrated by the presence in most species of a sulfate-containing carbohydrate HNK-1 epitope, which is expressed on a large number of neural adhesion proteins, including NCAM and MAG, and functions in cell—cell interactions. As with other proteins in the immunoglobulin superfamily, it is likely that the interaction of MAG with its ligand(s) on the axolemma mediates cell—cell signaling by mechanisms involving phosphorylation [19,26]. For example, the cytoplasmic domain of MAG interacts with fyn tyrosine kinase and phospholipase Cγ by mechanisms that appear to involve phosphorylated amino acids on MAG.
Although MAG is presumed to function in important signaling mechanisms between axons and oligodendrocytes during myelin formation, there may be some redundancy involved since young MAG knockout mice myelinate relatively normally and exhibit only subtle periaxonal structural abnormalities. However, as the null mutants age to 8 to 10 months, they develop a dying-back oligodendrogliopathy [27] and a peripheral neuropathy affecting both myelin and axons, suggesting that the most critical functions of MAG may be for maintenance of axon—myelin complexes. Also, although MAG generally has been thought to function in axon-to-glia signaling, the neuronal abnormalities found in aging MAG-null mutants suggest that it may also function in glia-to-axon signaling [26]. Furthermore, MAG is one of the factors in CNS white matter that inhibits neurite outgrowth in tissue culture [26]. Whatever the possible physiological implications of this for neuronal regeneration in vivo following injury, the findings are consistent with a MAG-mediated signaling mechanism from glia to axons that can affect the properties of neurons.
There are a large number of other glycoproteins associated with white matter and myelin, many of which have not yet been studied well. However, in addition to MAG, a few have been cloned and partially characterized. One of these is a minor protein of 26 kDa called the myelin-oligodendrocyte glycoprotein (MOG) [28]. MOG also is a transmembrane glycoprotein, contains a single immunoglobulin-like domain and one site for N-linked glycosylation and expresses the adhesion-related HNK-1 epitope. Unlike MAG, which is sequestered at the interior of myelin sheaths, MOG is localized on the surface of myelin sheaths and oligodendrocytes. Because of its surface location, it may function in transmitting extracellular information to the interior of oligodendrocytes and has been implicated as a target antigen in autoimmune aspects of demyelinating diseases of the CNS (see Chap. 39).
There is also a 120-kDa glycosylated protein in white matter called the oligodendrocyte-myelin glycoprotein [29]. This glycoprotein is membrane-bound through a phosphatidylinositol linkage and characterized by a cysteine-rich motif at the N-terminus and a series of tandem leucine-rich repeats. Unlike MAG and MOG, it is not a member of the immunoglobulin superfamily but does express the HNK-1 carbohydrate epitope. The leucine-rich repeats and adhesion-related HNK-1 epitope suggest that it also may function in cell—cell interactions.
Small amounts of proteins characteristic of membranes in general can be identified on gels of myelin proteins. Noted in Figure 4-11 is tubulin; although this may be present because of contamination of myelin preparation by other membranes, there is evidence suggesting that it is an authentic myelin component. High-resolution electrophoretic techniques demonstrate the presence of other minor protein bands; these may relate to the presence of numerous enzyme activities associated with the myelin sheath (see below).
Peripheral myelin contains some unique proteins and some shared with central nervous system myelin
P0 is the major PNS myelin protein. Gel-electrophoretic analysis (Fig. 4-11A, C) shows that a single protein, of 30 kDa, P0 accounts for more than half of the PNS myelin protein. The cloning and sequencing of the message for this protein [30, 31] led to derivation of amino acid sequences from several species. From this, it has been deduced that the protein has about 220 amino acids with an intracellular domain, a hydrophobic transmembrane domain and a single extracellular immunoglobulin-like domain. The amino-terminal extracellular domain includes a signal sequence for insertion of protein into the membrane and a glycosylation site. In addition to the well-characterized carbohydrate chain, other post-translational modifications include sulfation, phosphorylation and acylation.
It is interesting to note that PLP and P0 protein, although different in sequence, post-translational modifications and structure, may have similar roles in the formation of structures as closely related as myelins of the CNS and PNS. These proteins are not mutually exclusive; they are coexpressed in certain fish and amphibians [32]. Transfection of non-neural cells with the P0 gene results in cell—cell interaction, which can be demonstrated to be due to homophilic interactions of the extracellular domains of P0 [33,34]. Elucidation of the crystal structure of the extracellular domain of P0 shows tetrameric packing of P0 molecules, suggesting that the extracellular domains of P0 project from the myelin membrane surface as tetramers [35]. The complete knockout of P0 has profound consequences on myelin structure and function [36], in contradistinction to the previously noted, relatively benign consequences for CNS in animals with a deletion of the PLP gene.
Myelin basic protein content in the PNS varies from approximately 5 to 18% of total protein, in contrast to the CNS, where it is on the order of 30%. In rodents, the same four MBPs found in the CNS are present in the PNS, with molecular weights of 21,000, 18,500, 17,000 and 14,000 respectively. In adult rodents, the 14-kDa MBP is the most prominent component and is termed “Pr” in the PNS nomenclature. The 18-kDa component is present and often is referred to as the P1 protein in the nomenclature of peripheral myelin proteins. Another species-specific variation occurs in humans. In human PNS, the major basic protein is not the 18.5-kDa form, which is most prominent in the CNS, but rather the 17.2-kDa form [11]. MBP may not play as critical a role in myelin structure in the PNS as it does in the CNS. The murine mutant Shiverer is lesioned with respect to MBP synthesis. CNS myelin has no dense line structure; this contrasts to the PNS, which has almost normal myelin amount and structure but lacks MBP.
PNS myelin contains another positively charged protein, referred to as P2, with Mr ~15,000. It is unrelated in sequence to either P1 or Pr but shows strong homology to a family of cytoplasmic lipid-binding proteins that are present in a variety of cell types [37]. This suggests the possibility of P2 protein involvement in lipid assembly or turnover within the myelin sheath. The amount of P2 protein is highly variable from species to species, accounting for about 15% of total protein in bovine PNS myelin, 5% in humans and less than 1% in rodents. Within a species, P2 is more prominent in the thicker myelin sheaths. P2 protein generally is considered in the context of PNS myelin proteins, but it is expressed in small amounts of CNS myelin sheaths of some species. P2 is an antigen for experimental allergic neuritis (EAN), the PNS counterpart of EAE (see Chap. 39). P2 appears to be present in the major dense line of myelin sheaths, where it may play a structural role similar to MBP, and there appears to be substantially more P2 in large sheaths than small ones. The large variation in the amount and distribution of the protein from species to species and sheath to sheath raises questions about its function. Its similarities to cytoplasmic proteins, whose functions appear to involve solubilization and transport of fatty acids and retinoids, suggest that it might function similarly in myelination; but there is currently no experimental evidence to support this hypothesis.
Other glycoproteins of PNS myelin. In addition to the major P0 glycoprotein, compact PNS myelin contains a 22-kDa glycoprotein called peripheral myelin protein-22 (PMP-22) [38], which accounts for less than 5% of the total protein (Fig. 4-11C). PMP-22 has four potential transmembrane domains and a single site for N-linked glycosylation. It is referred to as a growth arrest protein because its cDNA was cloned from nondividing fibroblasts and the synthesis of PMP-22 and other myelin proteins ceases when Schwann cells begin to proliferate following nerve transection. Since it is quantitatively a rather minor component, it may perform some unknown dynamic function in myelin assembly or maintenance rather than a major structural role. Its putative tetraspan structure is similar to that of PLP, suggesting that its role might be one of the functions of PLP in CNS myelin. Also, as is the case with PLP, any significant deviation in gene dosage for PMP-22 or disruption caused by point mutations has severe functional consequences [39]. Abnormalities of the PMP-22 gene are responsible for the trembler murine mutant and several inherited human neuropathies (see Chap. 39).
Similarly to the CNS, MAG is present in the periaxonal membranes of the myelin-forming Schwann cells, but it is also present in the Schwann cell membranes constituting the Schmidt-Lentermann incisures, paranodal loops and outer mesaxon [26]. All of these locations are characterized by 12- to 14-nm spacing between the extracellular surfaces of adjacent membranes and the presence of cytoplasm on the inner side of the membranes. Therefore, in addition to a role in Schwann cell—axon interactions in the PNS, MAG may function in interactions between adjacent Schwann cell membranes at the other locations. Both isoforms of MAG are present in the PNS of rodents, although S-MAG is the predominant isoform at all ages. As mentioned earlier, the peripheral neuropathy affecting both myelin and axons that develop in aging MAG knockout mice suggests that MAG-mediated signaling from axons to Schwann cells, and vice versa may be important for the maintenance of myelin—axon complexes. Clinical interest in PNS MAG derives from the demonstration that human IgM monoclonal antibodies in patients with neuropathy in association with gammopathy react with a carbohydrate structure in MAG that is very similar to the adhesion-related HNK-1 carbohydrate epitope (see Chap. 39).
PNS myelin also contains a glycoprotein of 170 kDa that accounts for about 5% of the total myelin protein and appears to be the same as a protein that was characterized further and called the Schwann cell membrane glycoprotein (SAG) [40]. It appears to be expressed in locations distinct from compact myelin by both myelinating and nonmyelinating Schwann cells, but very little is known about its structure and function at this time. In addition, avian Schwann cells contain a glycoprotein with relatively high amino acid sequence homology to MAG, the Schwann cell myelin protein (SMP) [26]. Although it is not thought to be the avian homolog of MAG because of differences in its pattern of expression and less than expected sequence homology, the structural and functional relationships between SMP and MAG remain to be established.
Myelin contains enzymes that function in metabolism and possibly ion transport
Several decades ago, it was generally believed that myelin was an inert membrane that did not carry out any biochemical functions. More recently, however, a large number of enzymes have been discovered in myelin [41]. These findings imply that myelin is metabolically active in synthesis, processing and metabolic turnover of some of its own components. Additionally, it may play an active role in ion transport with respect to not only maintenance of its own structure but also participation in buffering of ion levels in the vicinity of the axon.
A few enzymes, such as the previously mentioned CNP, are believed to be fairly myelin-specific, although they are probably also present in oligodendroglial membranes. CNP is very low in peripheral nerve and PNS myelin, suggesting some function more specialized to the CNS. A pH 7.2 cholesterol ester hydrolase also may be relatively myelin-specific, although the enzyme is prominent in myelin. N-Acetyl-l-aspartate aminohydroxylase, an enzyme operating on a substrate of unknown metabolic significance, also is enriched in myelin.
There are many enzymes that are not myelin-specific but appear to be intrinsic to myelin and not contaminants. Several proteolytic activities have been identified in purified myelin; the presence of neutral protease activity is well documented. The presence in myelin of cAMP-stimulated kinase, calcium/calmodulin-dependent kinase and protein kinase C activities has been reported. Phosphoprotein phosphatases are also present. Protein kinase C and phosphatase activities are presumed to be responsible for the rapid turnover of phosphate groups of MBP. Enzyme activity for acylation of PLP is also intrinsic to myelin.
Enzymes involved in the metabolism of structural lipids include a number of steroidmodifying and cholesterol-esterifying enzymes, UDP-galactose:ceramide galactosyltransferase and many enzymes of glycerophospholipid metabolism (see Chap. 3). The latter grouping includes all of the enzymes necessary for phosphatidyl ethanolamine synthesis from diradyl-sn-glycerol and ethanolamine; it is likely that phosphatidycholine also can be synthesized within myelin. Perhaps even more elemental building blocks can be assembled into lipids by myelin enzymes. Acyl-CoA synthetase is present in myelin, suggesting the capacity to integrate free fatty acids into myelin lipids. The extent of the contribution of these enzymes in myelin, relative to enzymes within the oligodendroglial perikaryon, to metabolism of myelin lipids is not known.
Other enzymes present in myelin include those involved in phosphoinositide metabolism: phosphatidylinositol kinase, diphosphoinositide kinase, the corresponding phosphatases and diglyceride kinases. These are of interest because of the high concentration of polyphosphoinositides of myelin and the rapid turnover of their phosphate groups. This area of research has expanded toward characterization of a signal-transduction system(s). There is evidence for the presence in myelin of muscarinic cholinergic receptors, G proteins, phospholipases C and D and protein kinase C.
Certain enzymes present in myelin could be involved in ion transport. Carbonic anhydrase generally has been considered a soluble enzyme and a glial marker, but myelin accounts for a large part of the membrane-bound form in brain. This enzyme may play a role in removal of carbonic acid from metabolically active axons. The enzymes 5′-nucleotidase and Na,K-ATPase have long been considered specific markers for plasma membranes and are found in myelin at low concentrations. The 5′-nucleotidase activity may be related to a transport mechanism for adenosine, and Na,K-ATPase could function in transport of monovalent cations. The presence of these enzymes suggests that myelin may have an active role in transport of material in and out of the axon. In connection with this hypothesis, it is of interest that the PLP gene family may have evolved from a pore-forming polypeptide [15]. K+ channels in myelin vesicles also have been described [42]. An isoform of glutathione-S-transferase is present in myelin and may be involved in transport of certain larger molecules.
- Central nervous system myelin is enriched in certain lipids
- Peripheral and central nervous system myelin lipids are qualitatively similar
- Central nervous system myelin contains some unique proteins
- Peripheral myelin contains some unique proteins and some shared with central nervous system myelin
- Myelin contains enzymes that function in metabolism and possibly ion transport
- Characteristic Composition of Myelin - Basic NeurochemistryCharacteristic Composition of Myelin - Basic Neurochemistry
Your browsing activity is empty.
Activity recording is turned off.
See more...