All material appearing in this report is in the public domain and may be reproduced or copied without permission from SAMHSA. Citation of the source is appreciated. However, this publication may not be reproduced or distributed for a fee without the specific, written authorization of the Office of Communications, SAMHSA, HHS.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013-.
Summary
Background: Opioid addiction is a serious concern in the United States that can be treated successfully through medication-assisted treatment (MAT). Methadone, buprenorphine, and extended-release, injectable naltrexone are three medications that have been approved by the FDA for treatment of opioid use disorder. Method: National Survey of Substance Abuse Treatment Services data for years 2003 through 2015 were used to assess the usage and trends of MAT in facilities with and without OTPs. Results: This analysis found that the numbers of facilities utilizing MAT for opioid use disorder and the numbers of clients receiving each of the three medications has increased from 2003 to 2015. Conclusion: The increases in numbers of facilities providing MAT services and the increase in numbers of clients receiving MAT is an indication that facilities are responding to the demand for effective treatment for opioid use disorder. However, without a leveling off in the trends, there is indication of unmet need. Public health resources and education could be used to further increase access to treatment and inform the public about treatment options.
Keywords:
Opioid use disorder, Methadone, Buprenorphine, Naltrexone, Medication-assisted Treatment (MAT), Opioid Treatment Program (OTP)In Brief
- Opioid Treatment Programs (OTPs) are regulated by the Substance Abuse and Mental Health Services Administration (SAMHSA) and are qualified to dispense the controlled substances, methadone and buprenorphine, to treat addiction to opioids (e.g., heroin and prescription pain relievers).
- The number of OTPs has increased from approximately 1,100 in 2003 to almost 1,500 by the end of 2016 and the number of clients receiving methadone on the survey reference date increased from about 227,000 in 2003 to over 350,000 in 2015.
- The percentage of OTPs offering buprenorphine increased from 11 percent in 2003 to 58 percent in 2015; the percentage of facilities without OTPs offering buprenorphine increased from 5 percent in 2003 to 21 percent in 2015.
- The percentage of OTPs offering extended-release, injectable naltrexone increased from 11 percent in 2011 to 23 percent in 2015; the percentage of facilities without OTPs offering extended-release, injectable naltrexone increased from 8 percent in 2011 to 16 percent in 2015.
Introduction
In 2015, an estimated 2.4 million people in the United States had a past year opioid use disorder which includes heroin and prescription opioids such as oxycodone and hydrocodone.1 Withdrawal from these drugs is generally so intense that those with opioid use disorder continue taking the drugs in increasing dosages to avoid the withdrawal. Withdrawal symptoms may begin within 4 to 6 hours of the last heroin usage and may last for up to several months.2
An effective treatment for opioid use disorder includes medication-assisted treatment (MAT) which combines behavioral therapy and medications. The Food and Drug Administration (FDA) approved medications for use in treating opioid use disorder include methadone, buprenorphine (buprenorphine with naloxone), and naltrexone. Naltrexone is an opioid antagonist, methadone is an opioid agonist and buprenorphine is a partial opioid agonist.3,4 MAT has been found to reduce morbidity and mortality, decrease overdose deaths, reduce transmission of infectious disease, increase treatment retention, improve social functioning, and reduce criminal activity.5
Methadone, in use since 1964 used for the treatment of opioid use disorder, may be dispensed only in federally-approved Opioid Treatment Programs (OTPs). Treatment protocols require that a client take the medication at the clinic where it is dispensed daily.6 Take-home dosages generally are allowed only for clients who have been on an established maintenance program for an extended period of time.
In October 2002, buprenorphine was approved by the Food and Drug Administration (FDA) for the treatment of opioid use disorder. Physicians who obtain specialized training may prescribe buprenorphine. Some of these physicians are private, office-based practices; others are affiliated with substance abuse treatment facilities or programs and may prescribe buprenorphine to clients at those facilities. Additionally, OTPs may also prescribe and/or dispense buprenorphine. In October 2010, extended-release, injectable naltrexone was approved by the FDA to treat and prevent relapse in clients with opioid use disorder following medical withdrawal management from opioids. Extended-release injectable naltrexone may be prescribed by any individual who is licensed to prescribe medication (e.g., physician, physician assistant, nurse practitioner) or order administration by qualified staff.
Methods
The National Survey of Substance Abuse Treatment Services (N-SSATS), an annual, national survey of all known substance abuse treatment facilities, both public and private, provides information on the numbers of facilities that provide medication-assisted treatment with methadone, buprenorphine, and/or naltrexone, as well as on the numbers of clients receiving these medications. This report updates the trends7 in the use of methadone and buprenorphine, and adds to those trends by including the use of extended-release, injectable naltrexone in the treatment of opioid use disorder in substance abuse treatment facilities. This report includes data from OTPs as well as facilities that did not have OTPs (hereafter referred to as “non-OTP facilities”). It does not include data from private physicians who are not affiliated with a substance abuse treatment program or facility.
Methadone
In 2015, 10 percent of all substance abuse treatment facilities had OTPs (Figure 1). This percentage consistently has been between 8 to 10 percent since 2001, when the Substance Abuse and Mental Health Services Administration began certifying OTPs. The number of facilities with OTPs remained relatively constant at around 1,100 to 1,200 facilities between 2003 and 2012. However, a recent increase in the past few years to 1,482 OTPs on December 17, 2016, is evident.8 The number of clients receiving methadone on the survey reference date9 has steadily increased from about 227,000 in 2003 to 356,843 in 2015 (Figure 2). Clients receiving treatment with methadone accounted for approximately 21 to 25 percent of all substance abuse treatment clients each year. The increase in the number of clients receiving methadone treatment coupled with the stability of the proportion of clients receiving this treatment indicates that the overall availability of methadone treatment has increased over time.

Figure 1
Number of Opioid Treatment Programs (OTPs) and Percentage of Total Substance Abuse Treatment Facilities that Provided Them: 2003 to 2015.

Figure 2
Figure 2. Number of Opioid treatment Programs (OTP) Clients Receiving Methadone: 2003 to 2015.
Buprenorphine
With the introduction of buprenorphine at the end of 2002, OTPs and non-OTP facilities with a specially trained physician on staff began offering MAT for opioid use disorder with buprenorphine. The number of OTPs offering buprenorphine increased from 11 percent of OTPs in 2003 (121 OTPs) to 58 percent of OTPs in 2015 (779 OTPs) (Figure 3). Among non-OTP facilities, in 2003, about 5 percent (620 facilities) offered buprenorphine services; by 2015, the percentage of non-OTP facilities that offered buprenorphine services had increased to 21 percent (2,625 facilities) (Figure 4).

Figure 3
Number and Percentage of Opioid Treatment Programs (OTPs) Providing Buprenorphine and Extended Release Naltrexone: 2003 to 2015.
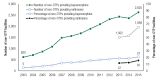
Figure 4
Number and Percentage of Facilities without Opioid Treatment Programs (non-OTPs) Providing Buprenorphine and Extended Release Naltrexone: 2003 to 2015.
Likewise, the numbers of clients receiving buprenorphine at substance abuse treatment facilities on the survey reference date increased during this period. At OTPs, the number of clients increased from 727 clients in 2004, the first year N-SSATS collected buprenorphine client counts, to 21,236 clients in 2015; at non-OTPs, the number increased from 1,670 clients in 2004 to 54,488 clients in 2015 (Figure 5). These buprenorphine numbers include only those clients who received their buprenorphine through a DATA 2000 waivered physician affiliated with a facility; it does not include any clients who received buprenorphine through an independent DATA 2000 waivered physician.
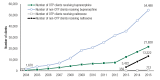
Figure 5
Number of Clients Receiving Buprenorphine or Extended Release Naltrexone at Opioid Treatment Programs (OTPs) and in Facilities without OTPs (non-OTPs): 2004 to 2015.
Extended-Release Injectable Naltrexone
Extended-release injectable naltrexone for the use of relapse prevention in the treatment of opioid use disorder was approved in late 2010. In 2011, facilities were first asked in N-SSATS if they provided extended-release, injectable naltrexone services. A total of 11 percent (125) of facilities with OTPs and 8 percent (968) of facilities without OTPs offered extended-release, injectable naltrexone services in 2011, whereas 23 percent (315) of facilities with OTPs and 16 percent (1,959) of facilities without OTPs offered these services in 2015.
N-SSATS first collected client counts for extended-release, injectable naltrexone usage in 2013. In 2013, 359 clients in facilities with OTPs and 3,422 clients in facilities without OTPs received extended-release, injectable naltrexone services, and in 2015, a total of 712 clients in facilities with OTPs and 6,323 clients in facilities without OTPs received these services (Figure 5). Again, these numbers include only those clients who received their naltrexone services through a treatment facility, not though an independent medical professional.
Discussion
Opioid use disorder, whether to heroin or prescription pain relievers, is a serious, life-threatening medical condition. Methadone and buprenorphine are medications that permit individuals with addiction to function normally within their families, jobs, and communities. While treatment with methadone is more established, it requires daily visits to an OTP. Not all individuals who could benefit from methadone treatment live within easy travelling distance of an OTP. Furthermore, the requirement for daily visits can interfere with jobs and other important activities. The use of buprenorphine for the treatment of opioid use disorder provides an alternative to methadone treatment for some individuals; however, buprenorphine may not be appropriate for all persons with opioid addiction. The dramatic increase in the number of clients receiving buprenorphine through treatment facilities is an indication of the demand for safe and effective medications for the treatment of opioid use disorder in the context of a broader treatment program. Furthermore, the introduction of extended-release, injectable naltrexone further increases the opportunities for those with opioid use disorder on their paths toward recovery.
Suggested Citation
Alderks, C.E., Trends in the Use of Methadone, Buprenorphine, and Extended-release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (Update). The CBHSQ Report: August 22, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed: 29200242]
Endnotes
- 1.
- Substance Abuse and Mental Health Services Administration. (2015). National Survey on Drug Use and Health. Unpublished table. Rockville, MD. [PubMed: 30199191]
- 2.
- Mental Health and Drug & Alcohol Office, New South Wales Department of Health. (2008). NSW drug and alcohol withdrawal clinical practice guidelines (SHPN [MHDAO] 070083). Retrieved from http://www1
.health.nsw .gov.au/pds/ActivePDSDocuments /GL2008_011.pdf - 3.
- An opioid agonist is a drug that activates the opioid (mu) receptors on nerve cells in the brain. A full agonist (methadone) continues to produce effects on the receptors until all receptors are fully activated or until the maximum effect is reached resulting in a relief of cravings, blocking of the euphoric effects associated with heroin and other opioids, and preventing withdrawal. A partial agonist (such as buprenorphine) activates the mu receptors, but not to the same extent as a full agonist; the effects increase until a plateau is reached. Once a plateau is reached and maintained, those with opioid addiction will not experience withdrawal symptoms. An opioid antagonist (such as naltrexone) binds to the opioid receptors with greater affinity than agonists, but does not activate the receptors; they block the receptor; therefore, preventing the neurons from responding to opioids, effectively blocking the effects of opioids. The result is a reversal of the effects of opioids and is used in the management of opioid use disorder to aid in the prevention of relapse.
- 4.
- Center for Substance Abuse Treatment. (2005). Medication-assisted treatment for opioid addiction in opioid treatment programs. Treatment Improvement Protocol (TIP) Series 43 (Rev. ed.; HHS Publication No. SMA 12–4214). Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed: 22514849]
- 5.
- Volkow, N. D., Frieden, T. R., Hyde, P. S., & Cha, S. S. (2014). Medication-assisted therapies—tackling the opioid-overdose epidemic. The New England Journal of Medicine, 370(22), 2063–2066. 10.1056/NEJMp1402780 [PubMed: 24758595] [CrossRef]
- 6.
- Maintenance treatment involves the substitution of a long-acting orally administered opioid, such as methadone, for the shorter-acting opioids, such as heroin, that are usually injected. Because methadone is long-acting, it may be taken once a day. It eliminates withdrawal symptoms for 24 to36 hours.
- 7.
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. (April 23, 2013). The N-SSATS Report: Trends in the Use of Methadone and Buprenorphine at Substance Abuse Treatment Facilities: 2003–2011. Rockville, MD.
- 8.
- The increase in the number of OTPs reporting to the N-SSATS appears to be a combination of an actual increase in OTPs coupled with better identification of OTPs in the survey and response to the survey. As N-SSATS is a voluntary survey, not all OTPs respond to the N-SSATS; numbers reported in this report include only those OTPs that responded to the N-SSATS. The actual number of OTPs on December 17, 2016 (1,482 OTPs) was obtained from the master list of certified OTPs maintained by the Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration.
- 9.
- The number of clients receiving methadone, buprenorphine, or injectable naltrexone on the survey reference date refers to the number of clients receiving methadone, buprenorphine, or extended-release, injectable naltrexone at a residential or hospital inpatient facility on the date of the last weekday in March and the number of clients who received methadone, buprenorphine, or extended release naltrexone at an outpatient facility during the month of March and who were still enrolled in treatment on the last weekday of March.
- PubMedLinks to PubMed
- Review Trends in the Use of Methadone and Buprenorphine at Substance Abuse Treatment Facilities: 2003 to 2011.[The CBHSQ Report. 2013]Review Trends in the Use of Methadone and Buprenorphine at Substance Abuse Treatment Facilities: 2003 to 2011.Alderks CE. The CBHSQ Report. 2013
- Characteristics and current clinical practices of opioid treatment programs in the United States.[Drug Alcohol Depend. 2019]Characteristics and current clinical practices of opioid treatment programs in the United States.Jones CM, Byrd DJ, Clarke TJ, Campbell TB, Ohuoha C, McCance-Katz EF. Drug Alcohol Depend. 2019 Dec 1; 205:107616. Epub 2019 Oct 17.
- Court personnel attitudes towards medication-assisted treatment: A state-wide survey.[J Subst Abuse Treat. 2019]Court personnel attitudes towards medication-assisted treatment: A state-wide survey.Andraka-Christou B, Gabriel M, Madeira J, Silverman RD. J Subst Abuse Treat. 2019 Sep; 104:72-82. Epub 2019 Jun 28.
- Differences in Availability and Use of Medications for Opioid Use Disorder in Residential Treatment Settings in the United States.[JAMA Netw Open. 2020]Differences in Availability and Use of Medications for Opioid Use Disorder in Residential Treatment Settings in the United States.Huhn AS, Hobelmann JG, Strickland JC, Oyler GA, Bergeria CL, Umbricht A, Dunn KE. JAMA Netw Open. 2020 Feb 5; 3(2):e1920843. Epub 2020 Feb 5.
- Review Medications for Treatment of Opioid Use Disorder among Persons Living with HIV.[Curr HIV/AIDS Rep. 2019]Review Medications for Treatment of Opioid Use Disorder among Persons Living with HIV.Fanucchi L, Springer SA, Korthuis PT. Curr HIV/AIDS Rep. 2019 Feb; 16(1):1-6.
- Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone a...Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (Update) - The CBHSQ Report
Your browsing activity is empty.
Activity recording is turned off.
See more...
