This book is copyright. Apart from any fair dealing for the purposes of private study, research, criticism or review as permitted under the Copyright Act, no part may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission. Address all inquiries to the Director at the above address.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011.
Abstract
Magnesium plays an important role in the prevention of central sensitization and in the attenuation of established pain hypersensitivity, and its main mode of action appears to involve its voltage-gated antagonist action at N-methyl-D-aspartate (NMDA) receptors. Given the putative function of the NMDA receptor in pain transduction, magnesium has been investigated in various clinical conditions associated with acute or chronic pain. The parenteral administration of magnesium, via an intravenous, intrathecal, or epidural route, may reduce pain, and anaesthetic and analgesic requirements during the intra- and post-operative periods. The beneficial effects of magnesium treatment have also been demonstrated in patients suffering from neuropathic pain, such as in those with malignancy-related neurologic symptoms, postherpetic neuralgia, diabetic neuropathy, and chemotherapy-induced peripheral neuropathy. In addition, magnesium therapy has been shown to be effective in alleviating dysmenorrhea, headaches, and acute migraine attacks. Magnesium is playing an evolving role in pain management, but a more thorough understanding of the mechanisms underlying its antinociceptive action and additional clinical studies are required to clarify its role as an analgesic adjuvant.
Introduction
The research interest in NMDA receptors has led to an examination of the interactions between NMDA receptors and the induction and maintenance of central sensitization after nociceptive stimuli (Woolf and Thompson, 1991). Ketamine and magnesium are representative NMDA receptor antagonists, and in particular, magnesium can regulate calcium access into cells by antagonizing the NMDA receptor (Paoletti and Neyton, 2007), which has encouraged investigations on the use of magnesium as an analgesic adjuvant. Recent studies have proposed a role for NMDA receptor antagonists in the management of postoperative pain and in other acute and chronic pain conditions. This chapter describes the pharmacologic basis of pain relief by the magnesium ion, and surveys various clinical studies that have examined the antinociceptive effects of magnesium.
Mechanism of magnesium as an analgesic adjuvant
Although magnesium has no direct analgesic effect, it inhibits calcium ions entering cells by blocking NMDA receptors, which causes an antinociceptive effect. Furthermore, this antinociceptive effect is related to its prevention of central sensitization caused by peripheral tissue injury (Woolf and Thompson, 1991). Central sensitization is the result of the enhancement of neuronal properties in nociceptive pathways of the central nervous system, and is triggered by repetitive nociceptive afferent inputs, which manifests as a prolonged reduction in the pain threshold. Central sensitization produces pain hypersensitivity, such as wind-up or long-term potentiation of pain, that is, it causes pain even when peripheral stimuli are not intense and continues to cause pain after the initiating stimuli have disappeared (Latremoliere and Woolf, 2009; Woolf, 1983; Woolf and Salter, 2000).
Increased intracellular calcium levels seem to play a major role in the initiation of central sensitization (Pockett, 1995; Woolf and Chong, 1993), and the build-up of intracellular calcium is associated with various receptors on postsynaptic neurons of the spinal dorsal horn, such as, NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), kainate, and glutamate receptors (Latremoliere and Woolf, 2009). Of these receptors, NMDA receptor activation has been demonstrated to be essential for initiating and maintaining central sensitization.
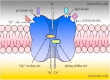
The NMDA receptor is a membrane ion channel expressed in the central nervous system. It is a tetramer composed of four different subunits, that is, two NR1 and two NR2 (Dingledine et al., 1999). NMDA receptors regulate the cellular inflows of Na+ and Ca2+, and the outflow of K+. This voltage-dependent ion channel is blocked non-competitively in the resting state by the magnesium ion and by ketamine (phencyclidine site blockade), MK-801, memantine, and others (Felsby et al., 1996; Paoletti and Neyton, 2007) (Fig. 1). On the other hand, the NMDA receptor channel is opened by membrane depolarization induced by the sustained release of glutamate and neuropeptides, which include substance P and calcitonin gene-related peptide (CGRP) (Baranauskas and Nistri, 1998; Mayer et al., 1984).
Extracellular magnesium blocks the NMDA receptor in a voltage-dependent manner (Mayer et al., 1984), and thus, can prevent the establishment of central sensitization and abolish existing hypersensitivity. Other noncompetitive or competitive NMDA receptor antagonists, such as MK801 and D-CPP, also prevent and reverse the hyperexcitability of neurons produced by nociceptive afferent inputs (Ma and Woolf, 1995; Woolf and Thompson, 1991).
Perioperative pain
Many authors have investigated the adjuvant role of magnesium in the context of intra- and post- operative analgesia. Magnesium has been shown to be effective for treating intra- and post- operative pain and for blunting autonomic, somatic, and endocrine reflexes to noxious stimuli (Kara et al., 2002; Koinig et al., 1998; Levaux et al., 2003). Usually, magnesium is administered as an i.v. 30-50 mg/kg bolus of magnesium sulphate as a loading dose, and maintained at 6-20 mg/kg/h by continuous infusion until the end of surgery (Koinig et al., 1998; Ryu et al., 2008; Ryu et al., 2009; Wilder- Smith et al., 1997), or for 4 hours after the initial bolus (Seyhan et al., 2006).
Tramer et al., (1996) conducted the first prospective, randomized study on the effect of magnesium on analgesic requirements, pain, comfort, and quality of sleep during the immediate postoperative period. They showedthat magnesium sulphate reduces analgesic requirements and discomfort, and improves quality of sleep during the postoperative period, and that it does not cause any adverse effect at 48 h after surgery. Oguzhan et al., (2008) studied the effect of a magnesium sulphate infusion on postoperative requirements for opioids, intraoperative muscle relaxant, inhalational anaesthetic consumption, and post-operative pain during and after lumbar disc surgery. Their results suggested that intraoperative magnesium administration significantly reduced intraoperative muscle relaxant and opioid requirements, and also reduced postoperative pain and opioid use.
When used during a variety of surgeries, magnesium was also found to reduce the need for intraoperative anaesthetics and muscle relaxants and to reduce the amount of morphine required to treat postoperative pain. Ryu et al., (2009) compared remifentanil and magnesium during middle ear surgery in terms of postoperative pain and other complications. In this study, magnesium or remifentanil combined with sevoflurane provided adequate hypotensive anaesthesia, but patients in the magnesium group experienced a more comfortable postoperative course with better analgesia, lessshivering, and less postoperative nausea and vomiting (PONV). Furthermore, the amount of sevoflurane required to maintain surgical anaesthesia was significantly lower in the magnesium group than in the remifentanil group (Ryu et al., 2009).
The use of magnesium as an analgesic adjunct has also been found to be beneficial in patients on total intravenous anaesthesia (TIVA). Significant reductions in intraoperative propofol, atracurium, and postoperative morphine consumption were observed in patients undergoing gynaecological surgery (Seyhan et al., 2006). In another study on gynaecologic patients receiving TIVA, post-operative pain scores, cumulative analgesic consumption, and shivering incidents were significantly lower in patients treated with magnesium, to the extent that the authors concluded magnesium sulphate during TIVA improved the quality of postoperative analgesia (Ryu et al., 2008). Furthermore, Choi et al., (2002) found that intravenous magnesium sulphate reduced propofol infusion requirements during the maintenance of propofol-nitrous oxide anaesthesia in patients undergoing total abdominal hysterectomy.
The usefulness of adjunctive magnesium for postoperative pain control has also been examined in the context of regional anaesthesia. Recent studies have suggested that magnesium may play a beneficial role in spinal anaesthesia. For example, magnesium was found to prevent the induction of central sensitization by peripheral nociceptive stimulation at a spinal site of action, by blocking NMDA receptors in a voltage-dependent fashion (Woolf and Thompson, 1991). Utilizing the same mechanism, the addition of small doses of magnesium sulphate to local anaesthetics for spinal anaesthesia enhances the duration of anaesthesia and reduces postoperative analgesic requirements and the incidence of side effects of high doses of local anaesthetics and opioids. In animal studies, the intrathecal co-administration of magnesium sulphate during spinal anaesthesia was found to significantly potentiate morphine analgesia in normal rats and in a surgical model of mechanical allodynia (Kroin et al., 2000). In another experimental model, intrathecal magnesium sulphate produced a state of spinal anaesthesia and general sedation in rats that lasted for around 1 h, and at 6 h post-injection, animals recovered and showed no evidence of neurotoxicity (Bahar et al., 1996). Clinical studies have also shown that intrathecal magnesium sulphate added to fentanyl prolongs analgesia without increasing side effects during labour analgesia (Buvanendran et al., 2002; Ozalevli et al., 2005). Furthermore, the i.v. infusion of magnesium sulphate during spinal anaesthesia was found to improve postoperative analgesia and reduce the cumulative consumption of analgesics after total hip replacement arthroplasty (Hwang et al., 2010). Postoperative adjunctive i.v. magnesium infusion was also found to increase time to analgesic need and to reduce total analgesia consumption after spinal anaesthesia (Apan et al., 2004).
Comparatively little is known of the effect of epidural magnesium sulphate, whereas intrathecal magnesium sulphate has been investigated on many occasions. Caudal epidural anaesthesia with lidocaine plus magnesium sulphate was found to produce analgesia of longer duration than lidocaine plus distilled water in cattle (Dehghani and Bigham, 2009). Arcioni et al., (2007) compared intrathecal, epidural, combined intrathecal and epidural magnesium sulphate, and spinal anaesthesia alone (controls) in patients undergoing orthopaedic surgery to investigate whether intrathecal and/or epidural magnesium sulphate could reduce postoperative analgesic requirements. The results obtained suggested that combined intrathecal and epidural magnesium sulphate significantly reduced postoperative analgesic requirements (Arcioni et al., 2007).
However, not all investigations have reported postoperative analgesic effects for magnesium sulphate. For example, perioperative i.v. magnesium infusion was not found to reduce postoperative pain or analgesic consumption in patients undergoing abdominal hysterectomy (Ko et al., 2001) or caesarean delivery (Paech et al., 2006). Furthermore, in a recent report issued by Tramer and Glynn (2007), pretreatment with magnesium sulphate was found to have no effect on postoperative pain or analgesic requirements over the first three postoperative days in patients undergoing ambulatory ilioinguinal hernia repair or varicose vein surgery. However, in this study, a single dose (4 g) of intravenous magnesium sulphate was used instead of a loading dose plus continuous infusion.
Although some debate exists concerning the role of magnesium sulphate as an analgesic adjuvant, the consensus is that magnesium sulphate acts to support a neuromuscular blockade. Magnesium acts as a calcium channel blocker at presynaptic nerve terminals and reduces acetylcholine release at the motor endplate (Fisher, 1999). This diminishes muscle fibre excitability and reduces end plate potential amplitudes, which leads to the potentiation of a neuromuscular blockade by nondepolarizing neuromuscular blockers (Fisher, 1999). Some authors have focused on the direct effect of magnesium on neuromuscular blockade (Fuchs-Buder et al., 1995; Fuchs-Buder and Tassonyi, 1996; Kussman et al., 1997; Ross and Baker, 1996; Telci et al., 2002), whereas others concluded that a perioperative adjuvant magnesium infusion enhances neuromuscular blockade (Lee and Kwon 2009; Oguzhan et al., 2008; Ryu et al., 2008; Ryu et al., 2009; Seyhan et al., 2006).
Controlled hypotension is sometimes required during surgery to maintain a bloodless operative field, and investigations on perioperative magnesium infusion for the control of hypotension during middle ear surgery decreased the incidences of postoperative nausea and vomiting (PONV) (Ryu et al., 2008; 2009), which could have been due to lower sevoflurane consumption (Apfel et al., 2002; Ryu et al., 2009) rather than any antiemetic effect of the magnesium. Nevertheless, since PONV is one of the most common and distressing complications after surgery, this effect is interesting, because it could be used to benefit, for example, patients undergoing ambulatory surgery. Perioperative i.v. magnesium infusion has another advantageous effect, in that it decreases the incidence of shivering by up to 70-90% (Ryu et al., 2008; Ryu et al., 2009; Tramer and Glynn, 2007). Shivering is one of the leading causes of postoperative discomfort (Alfonsi, 2001), and increases oxygen consumption (Alfonsi, 2001). Thus, the prevention of shivering is one of the most obvious benefits of magnesium sulphate administration in surgical patients.
When mixed with local anaesthetic, magnesium may also show beneficial effects in intravenous regional anaesthesia. In one study, magnesium was added to lidocaine for intravenous regional anaesthesia, and was found to improve quality of anaesthesia and analgesia, specifically, sensory and motor block onset times were shorter and postoperative analgesia was better with magnesium (Turan et al., 2005). However, in this study, recovery after intravenous regional anaesthesia was prolonged in the magnesium group (Turan et al., 2005). In contrast, i.v. magnesium infusion during general anaesthesia has not been found to delay recovery from anaesthesia in most investigations (Lee and Kwon, 2009; Ozcan et al., 2007; Ryu et al., 2008). Another consideration during magnesium administration is that magnesium may cause cardiovascular depression by acting as a calcium channel blocker. The consequent inhibition of catecholamine release lowers plasma epinephrine and norepinephrine concentrations after endotracheal intubation, and therefore reduces hypertensive responses during anaesthesia induction (James et al., 1989). Accordingly, magnesium should be used with caution in hypovolemic patients and in those with limited cardiac capacity.
Other pain conditions
Numerous clinical studies have found that magnesium has beneficial effects in patients suffering from neuropathic pain, dysmenorrhea, tension headache, acute migraine attack, and others. These effects are considered to be due to blockage of the NMDA receptor, attenuation of central sensitization, and muscle relaxing effects.
Neuropathic pain
Neuropathic pain is caused by damage to or by the dysfunction of a component of the peripheral or central nervous system (Backonja, 2003). Thus, the causes of neuropathic pain are various and include spinal cord injury, multiple sclerosis, diabetic neuropathy, radiation injury, complications of chemotherapy, a malignancy- related neurologic symptom, amongst others. Its common features include burning, a cold sensation, numbness, itching, and a shooting or abnormal sensation called dysesthesia in a continuous or episodic manner. The mechanism responsible for neuropathic pain remains unknown, but peripheral or central sensitization is a prime candidate (Bridges et al., 2001; Ossipov et al., 2000). Neuropathic pain is extremely difficult to treat, and is only partially relieved in some 40-60% of patients. Favoured pharmacologic treatments are antidepressants, anticonvulsants, opioids, and NMDA receptor antagonists (O'Connor and Dworkin, 2009). Magnesium has been shown to have beneficial effects on neuropathic pain in some clinical and animal studies. When magnesium sulphate (bolus doses of 500 mg or 1 g) was given intravenously to patients with uncontrolled neuropathic pain due to cancer infiltration, pain intensity and pain relief scores improved (Crosby et al., 2000). In another study, magnesium chloride (bolus 0.16 mmol/kg over 10 minutes followed by continuous infusion at 0.16 mmol/kg/h) reduced protracted pain and regions of allodynia in patients suffering from peripheral neuropathic pain (Felsby et al., 1996). In addition, in an animal model of neuropathic pain induced hyperalgesia subsided after intraperitoneal magnesium therapy (total 150 or 200 mg/kg for 5 days) (Begon et al., 2000).
Postherpetic neuralgia
Postherpetic neuralgia (PHN), a type of neuropathic pain, is characterized by chronic persistent pain after an acute herpes zoster attack (Rowbotham and Petersen, 2001). Typically, PHN causes a burning, aching, sharp, and lancinating pain that occurs in a continuous or paroxysmal pattern, and is often accompanied by hyperalgesia and allodynia. Brill et al., (2002) showed that magnesium can relieve hyperalgesia and allodynia in PHN. When they administered i.v. magnesium sulphate at 30 mg/kg over 30 min to patients with intractable PHN, pain was either relieved completely or diminished (Brill et al., 2002).
Diabetic neuropathy
Diabetes mellitus is the most common etiologic factor of peripheral neuropathy, and diabetic neuropathy is often expressed as numbness, a tingling sensation, and hypersensitivity to pain. These symptoms are limited to the fingers and toes during early disease, and then spread to the proximal extremities to produce a characteristic glove and stocking pattern (Head, 2006). Conventional treatments include antidepressants, anticonvulsants, lidocaine patches, opioid or non- opioids analgesics, and various alternative treatments (Head, 2006). Recently, dietary magnesium supplementation has been at the focus of attention, and oral doses of magnesium sulphate have been found to be effective at reducing thermal pain thresholds in rats. However, appropriate treatment dosages and durations have not been established (Hasanein et al., 2006). De Leeuw et al., (2004) also demonstrated that long-term magnesium supplementation (300 mg daily for 5 years) can favourably influence the progression of diabetic polyneuropathy.
Chemotherapy-induced peripheral neuropathy
CIPN is one of the most common side effects in cancer patients administered many chemo- therapeutic agents, such as cisplatin, 5- fluorouracil, vinca alkaloids, taxoids, and etoposide (Head, 2006; Hildebrand, 2006; Wolf et al., 2008). The symptoms and signs of CIPN may resolve completely or partially, but are sometimes irreversible (Kannarkat et al., 2007). Because the pathophysiology of CIPN has not been fully elucidated, various agents have been used to prevent or treat CIPN. Recently, it was reported that a Ca2+/Mg2+ infusion before and after oxaliplatin could prevent the development of CIPN (Gamelin et al., 2004), but the overall benefit of this is uncertain because of the different treatment efficacies of oxaliplatin and Ca2+/Mg2+ infusion combinations (Hochster et al., 2007). Furthermore, oxaliplatin metabolites are chelated by calcium and magnesium, and this could explain the observed neuroprotective effect. However, Bujalska et al., (2009) found that pretreatment with magnesium (30 mg/kg, i.p.) enhanced the analgesic effects of opioids in a vincristine-induced neuropathy model, and re- emphasized the antagonistic effect that magnesium has on NMDA receptors.
Dysmenorrhea
Dysmenorrhea refers to a gynaecological medical complaint characterized by severe uterine cramps associated with menstruation. Dysmenorrhea may precede or co-occur with menstruation, and causes a dull, nauseating, colicky to sharp or cramping pain. The overproduction of uterine prostaglandins or vasopressin, which stimulates myometrial contraction of the uterus, has been identified as a potential contributory factor (Stromberg et al., 1984; Woolf and Chong, 1993). When dysmenorrhea is severe enough to restrict daily activities, nonsteroidal anti-inflammatory drugs or oral contraceptive pills are required to reduce uterine contraction, and thus, relieve the pain (Harel, 2008). The benefits and safety of magnesium for the treatment of primary dysmenorrhea have also been reviewed (Bettendorf et al., 2008; Proctor and Murphy 2001), but more research is required to verify its efficacy. Briefly, oral magnesium on the day before menstruation and during the first and second days of the menstrual cycle for six consecutive cycles, was found to have a therapeutic effect on dysmenorrhea (Fontana- Klaiber and Hogg, 1990). However, the optimum treatment regimen has yet to be established and the mechanism responsible has not been determined. Nevertheless, it has been hypothesized that magnesium inhibits the biosynthesis of PGF2-α, which alleviates myometrial contraction and muscle relaxation by competing with calcium for entry into myometrial cells through voltage-gated channels (Seifert et al., 1989; Zahradnik and Breckwoldt, 1988).
Tension headache
Tension headaches are characterized by a diffuse, gradual, and pressure-like aching pain bilaterally over the top sides of the cranium (Altura and Altura, 2001). Its prevalence has been reported to range from around 10% to nearly 90% (Rasmussen et al., 1991; Robbins and Lipton, 2010; Russell et al., 2006). Although the exact aetiology of tension headaches is unknown, several pathophysiologies have been suggested, such as, muscle tension (Jensen and Rasmussen, 1996; Langemark and Olesen 1987), increased myofascial pain sensitivity (Pfaffenrath et al., 1998), platelet aggregation (Mishima et al., 1997), and others. Furthermore, in one study, it was shown that the magnesium ion levels of platelets were lower in patients with a tension headache (Mishima et al., 1997), and that magnesium supplementation appeared to ameliorate headaches, including the tension headaches (Mauskop et al., 1996; Thomas et al., 1994).
Conclusion
The postoperative analgesic adjuvant role of magnesium and its use as an analgesic therapy for the treatment of acute or chronic pain have been suggested for decades. Its antinociceptive effect has been suggested to be due to the blocking of NMDA receptors, and thus, the prevention of central sensitization. More consistent and convincing evidence is required before magnesium can be viewed as an effective adjuvant pain treatment.
References
- Alfonsi P. Postanaesthetic shivering: epidemiology, pathophysiology, and approaches to prevention and management. Drugs. 2001;61:2193–205. [PubMed: 11772130]
- Altura BM, Altura BT. Tension headaches and muscle tension: is there a role for magnesium? Med Hypotheses. 2001;57:705–13. [PubMed: 11918431]
- Apan A, Buyukkocak U, Ozcan S, Sari E, Basar H. Postoperative magnesium sulphate infusion reduces analgesic requirements in spinal anaesthesia. Eur J Anaesthesiol. 2004;21:766–9. [PubMed: 15678729]
- Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, Heineck R, Greim CA, Roewer N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–68. [PubMed: 12067003]
- Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romano S, Mercieri M, Masciangelo R, De Blasi RA, Pinto G. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce post-operative analgesic requirements: a prospective, randomized, double- blind, controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand. 2007;51:482–9. [PubMed: 17378788]
- Backonja MM. Defining neuropathic pain. Anesth Analg. 2003;97:785–90. [PubMed: 12933403]
- Bahar M, Chanimov M, Grinspun E, Koifman I, Cohen ML. Spinal anaesthesia induced by intrathecal magnesium sulphate. Anaesthesia. 1996;51:627–33. [PubMed: 8758153]
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol. 1998;54:349–65. [PubMed: 9481803]
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Res. 2000;887:436–9. [PubMed: 11134637]
- Bettendorf B, Shay S, Tu F. Dysmenorrhea: contemporary perspectives. Obstet Gynecol Surv. 2008;63:597–603. [PubMed: 18713479]
- Bridges D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth. 2001;87:12–26. [PubMed: 11460801]
- Brill S, Sedgwick PM, Hamann W, Di Vadi PP. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002;89:711–4. [PubMed: 12393768]
- Bujalska M, Makulska-Nowak H, Gumulka SW. Magnesium ions and opioid agonists in vincristine- induced neuropathy. Pharmacol Rep. 2009;61:1096–104. [PubMed: 20081245]
- Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trial. Anesth Analg. 2002;95:661–6. [PubMed: 12198056]
- Choi JC, Yoon KB, Um DJ, Kim C, Kim JS, Lee SG. Intravenous magnesium sulfate administration reduces propofol infusion requirements during maintenance of propofol-N2O anesthesia. Part I: comparing propofol requirements according to hemodynamic responses. Part II: comparing bispectral index in control and magnesium groups. Anesthesiology. 2002;97:1137–41. [PubMed: 12411798]
- Crosby V, Wilcock A, Corcoran R. The safety and efficacy of a single dose (500 mg or 1 g) of intravenous magnesium sulfate in neuropathic pain poorly responsive to strong opioid analgesics in patients with cancer. J Pain Symptom Manage. 2000;19:35–9. [PubMed: 10687324]
- De Leeuw I, Engelen W, De Block C, Van Gaal L. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg- depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109–14. [PubMed: 15319143]
- Dehghani SN, Bigham AS (2009) Comparison of caudal epidural anesthesia by use of lidocaine versus a lidocaine-magnesium sulfate combination in cattle. [PubMed: 19231950]
- Am J Vet Res. 70:194–7. [PubMed: 19231950]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed: 10049997]
- Felsby S, Nielsen J, Arendt-Nielsen L, Jensen TS. NMDA receptor blockade in chronic neuropathic pain: a comparison of ketamine and magnesium chloride. Pain. 1996;64:283–91. [PubMed: 8740606]
- Fisher DM. Clinical pharmacology of neuromuscular blocking agents. Am J Health Syst Pharm. 1999;56:S4–9. [PubMed: 10437710]
- Fontana-Klaiber H, Hogg B. Therapeutic effects of magnesium in dysmenorrhea. Schweiz Rundsch Med Prax. 1990;79:491–4. [PubMed: 2349410]
- Fuchs-Buder T, Wilder-Smith OH, Borgeat A, Tassonyi E. Interaction of magnesium sulphate with vecuronium-induced neuromuscular block. Br J Anaesth. 1995;74:405–9. [PubMed: 7734259]
- Fuchs-Buder T, Tassonyi E. Magnesium sulphate enhances residual neuromuscular block induced by vecuronium. Br J Anaesth. 1996;76:565–6. [PubMed: 8652332]
- Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, Gamelin E. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5- Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10:4055–61. [PubMed: 15217938]
- Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin Pharmacother. 2008;9:2661–72. [PubMed: 18803452]
- Hasanein P, Parviz M, Keshavarz M, Javanmardi K, Mansoori M, Soltani N. Oral magnesium administration prevents thermal hyperalgesia induced by diabetes in rats. Diabetes Res Clin Pract. 2006;73:17–22. [PubMed: 16417942]
- Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006;11:294–329. [PubMed: 17176168]
- Hildebrand J. Neurological complications of cancer chemotherapy. Curr Opin Oncol. 2006;18:321–4. [PubMed: 16721124]
- Hochster HS, Grothey A, Childs BH. Use of calcium and magnesium salts to reduce oxaliplatin- related neurotoxicity. J Clin Oncol. 2007;25:4028–9. [PubMed: 17664456]
- Hwang JY, Na HS, Jeon YT, Ro YJ, Kim CS, Do SH. (2010)
- I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth. 104:89–93. [PubMed: 19933175]
- James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg. 1989;68:772–6. [PubMed: 2735543]
- Jensen R, Rasmussen BK. Muscular disorders in tension-type headache. Cephalalgia. 1996;16:97–103. [PubMed: 8665589]
- Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20:719–25. [PubMed: 17992096]
- Kara H, Sahin N, Ulusan V, Aydogdu T. Magnesium infusion reduces perioperative pain. Eur J Anaesthesiol. 2002;19:52–6. [PubMed: 11913804]
- Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. [PubMed: 11575536]
- Koinig H, Wallner T, Marhofer P, Andel H, Horauf K, Mayer N. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87:206–10. [PubMed: 9661575]
- Kroin JS, McCarthy RJ, Von Roenn N, Schwab B, Tuman KJ, Ivankovich AD. Magnesium sulfate potentiates morphine antinociception at the spinal level. Anesth Analg. 2000;90:913–7. [PubMed: 10735798]
- Kussman B, Shorten G, Uppington J, Comunale ME. Administration of magnesium sulphate before rocuronium: effects on speed of onset and duration of neuromuscular block. Br J Anaesth. 1997;79:122–4. [PubMed: 9301400]
- Langemark M, Olesen J. Pericranial tenderness in tension headache. A blind, controlled study. Cephalalgia. 1987;7:249–55. [PubMed: 3427625]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. [PMC free article: PMC2750819] [PubMed: 19712899]
- Lee DH, Kwon IC. Magnesium sulphate has beneficial effects as an adjuvant during general anaesthesia for Caesarean section. Br J Anaesth. 2009;103:861–6. [PubMed: 19783538]
- Levaux C, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia. 2003;58:131–5. [PubMed: 12562408]
- Ma QP, Woolf CJ. Noxious stimuli induce an N- methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995;61:383–90. [PubMed: 7478681]
- Mauskop A, Altura BT, Cracco RQ, Altura BM. Intravenous magnesium sulfate rapidly alleviates headaches of various types. Headache. 1996;36:154–60. [PubMed: 8984087]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage- dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–3. [PubMed: 6325946]
- Mishima K, Takeshima T, Shimomura T, Okada H, Kitano A, Takahashi K, Nakashima K. Platelet ionized magnesium, cyclic AMP, and cyclic GMP levels in migraine and tension-type headache. Headache. 1997;37:561–4. [PubMed: 9385754]
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–32. [PubMed: 19801049]
- Oguzhan N, Gunday I, Turan A. Effect of magnesium sulfate infusion on sevoflurane consumption, hemodynamics, and perioperative opioid consumption in lumbar disc surgery. J Opioid Manag. 2008;4:105–10. [PubMed: 18557167]
- Ossipov MH, Lai J, Malan TP Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. [PubMed: 10911921]
- Ozalevli M, Cetin TO, Unlugenc H, Guler T, Isik G. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anaesthesia. Acta Anaesthesiol Scand. 2005;49:1514–9. [PubMed: 16223399]
- Ozcan PE, Tugrul S, Senturk NM, Uludag E, Cakar N, Telci L, Esen F. Role of magnesium sulfate in postoperative pain management for patients undergoing thoracotomy. J Cardiothorac Vasc Anesth. 2007;21:827–31. [PubMed: 18068060]
- Paech MJ, Magann EF, Doherty DA, Verity LJ, Newnham JP. Does magnesium sulfate reduce the short- and long-term requirements for pain relief after caesarean delivery? A double-blind placebo- controlled trial. Am J Obstet Gynecol. 2006;194:1596–602. [PubMed: 16615926]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. [PubMed: 17088105]
- Pfaffenrath V, Brune K, Diener HC, Gerber WD, Gobel H. The treatment of tension type headache. Headache. 1998;38:725–6.
- Pockett S. Spinal cord synaptic plasticity and chronic pain. Anesth Analg. 1995;80:173–9. [PubMed: 7802274]
- Proctor ML, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev. 2001:CD002124. [PubMed: 11687013]
- Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–57. [PubMed: 1941010]
- Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30:107–19. [PubMed: 20352581]
- Ross RM, Baker T. An effect of magnesium on neuromuscular function in parturients. J Clin Anesth. 1996;8:202–4. [PubMed: 8703454]
- Rowbotham MC, Petersen KL. Zoster-associated pain and neural dysfunction. Pain. 2001;93:1–5. [PubMed: 11406332]
- Russell MB, Levi N, Saltyte-Benth J, Fenger K. Tension-type headache in adolescents and adults: a population based study of 33,764 twins. Eur J Epidemiol. 2006;21:153–60. [PubMed: 16518684]
- Ryu JH, Kang MH, Park KS, Do SH. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br J Anaesth. 2008;100:397–403. [PubMed: 18276652]
- Ryu JH, Sohn IS, Do SH. Controlled hypotension for middle ear surgery: a comparison between remifentanil and magnesium sulphate. Br J Anaesth. 2009;103:490–5. [PubMed: 19687032]
- Seifert B, Wagler P, Dartsch S, Schmidt U, Nieder J. Magnesium--a new therapeutic alternative in primary dysmenorrhea. Zentralbl Gynakol. 1989;111:755–60. [PubMed: 2675496]
- Seyhan TO, Tugrul M, Sungur MO, Kayacan S, Telci L, Pembeci K, Akpir K. Effects of three different dose regimens of magnesium on propofol requirements, haemodynamic variables and postoperative pain relief in gynaecological surgery. Br J Anaesth. 2006;96:247–52. [PubMed: 16311277]
- Stromberg P, Akerlund M, Forsling ML, Granstrom E, Kindahl H. Vasopressin and prostaglandins in premenstrual pain and primary dysmenorrhea. Acta Obstet Gynecol Scand. 1984;63:533–8. [PubMed: 6542295]
- Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth. 2002;89:594–8. [PubMed: 12393361]
- Thomas J, Tomb E, Thomas E, Faure G. Migraine treatment by oral magnesium intake and correction of the irritation of buccofacial and cervical muscles as a side effect of mandibular imbalance. Magnes Res. 1994;7:123–7. [PubMed: 7999526]
- Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 1996;84:340–7. [PubMed: 8602664]
- Tramer MR, Glynn CJ. An evaluation of a single dose of magnesium to supplement analgesia after ambulatory surgery: randomized controlled trial. Anesth Analg. 2007;104:1374–9. [PubMed: 17513629]
- Turan A, Memis D, Karamanlioglu B, Guler T, Pamukcu Z. Intravenous regional anesthesia using lidocaine and magnesium. Anesth Analg. 2005;100:1189–92. [PubMed: 15781543]
- Wilder-Smith CH, Knopfli R, Wilder-Smith OH. Perioperative magnesium infusion and postoperative pain. Acta Anaesthesiol Scand. 1997;41:1023–7. [PubMed: 9311401]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15. [PubMed: 18571399]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–8. [PubMed: 6656869]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. [PubMed: 1828878]
- Woolf CJ, Chong MS. Preemptive analgesia-- treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. [PubMed: 8346839]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. [PubMed: 10846153]
- Zahradnik HP, Breckwoldt M. Drug therapy of dysmenorrhea. Gynakologe. 1988;21:58–62. [PubMed: 2897319]
- Review Magnesium and Pain.[Nutrients. 2020]Review Magnesium and Pain.Shin HJ, Na HS, Do SH. Nutrients. 2020 Jul 23; 12(8). Epub 2020 Jul 23.
- Review Management of pain using magnesium sulphate: a narrative review.[Postgrad Med. 2022]Review Management of pain using magnesium sulphate: a narrative review.Soleimanpour H, Imani F, Dolati S, Soleimanpour M, Shahsavarinia K. Postgrad Med. 2022 Apr; 134(3):260-266. Epub 2022 Feb 7.
- Magnesium in Pain Research: State of the Art.[Curr Med Chem. 2017]Magnesium in Pain Research: State of the Art.Srebro D, Vuckovic S, Milovanovic A, Kosutic J, Vujovic KS, Prostran M. Curr Med Chem. 2017; 24(4):424-434.
- Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain.[J Physiol. 2010]Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain.Rondón LJ, Privat AM, Daulhac L, Davin N, Mazur A, Fialip J, Eschalier A, Courteix C. J Physiol. 2010 Nov 1; 588(Pt 21):4205-15.
- Review Magnesium as an Alternative or Adjunct to Opioids for Migraine and Chronic Pain: A Review of the Clinical Effectiveness and Guidelines[ 2017]Review Magnesium as an Alternative or Adjunct to Opioids for Migraine and Chronic Pain: A Review of the Clinical Effectiveness and GuidelinesBanerjee S, Jones S. 2017 Apr 20
- The role of magnesium in pain - Magnesium in the Central Nervous SystemThe role of magnesium in pain - Magnesium in the Central Nervous System
Your browsing activity is empty.
Activity recording is turned off.
See more...