Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- OR
Odds ratio
- RCT
randomized controlled trial
- RD
risk difference
- RR
relative risk
Context and Policy Issues
Mucus secretion clearance is a defense mechanism used by the lung to protect itself from pathogens and particles present in the inhaled air.1,2 Mucus traps pathogens and particles in inhaled air, and is usually cleared from the lungs and airways by airflow and ciliary hairs.2 Impaired mucous clearance results in abnormal lung function.3
Mucus is a viscoelastic gel-like substance and consists of glycoproteins known as mucins, mixed with other proteins, lipids and water.2 In healthy individuals, mucus has low viscosity and elasticity and is easily cleared, however in certain lung diseases the mucus has higher viscosity and elasticity and is not easily cleared. Pharmacologic treatments for impaired mucous secretion clearance include agents such as isotonic saline, hypertonic saline, dornase alpha, and acetylcysteine (also known as N-acetylcysteine [NAC]). NAC hydrolyzes the disulfide bonds of mucus proteins to decrease mucus viscosity, thereby facilitating its clearance.4 NAC is used as a treatment option in various conditions in which there are problems with clearance of lung mucosal secretions (such as chronic obstructive pulmonary disease [COPD], chronic bronchitis, and intubated or post-operative patients).1,4–7
The purpose of this report is to review the comparative clinical effectiveness and safety of NAC for treating adult patients requiring mucous secretion clearance. Additionally, tfor this patient population, the clinical effectiveness of treatment with nebulized acetylcysteine versus oral acetylcysteine will be reviewed. A subsequent report will review the evidence-based guidelines regarding NAC for the treatment of adult patients requiring mucous secretion clearance.
Research Questions
What is the comparative clinical effectiveness of acetylcysteine versus other treatments for patients requiring mucous secretion clearance?
What is the evidence regarding the safety of acetylcysteine when used for patients requiring mucous secretion clearance?
What is the comparative clinical effectiveness of nebulized acetylcysteine versus oral acetylcysteine for patients requiring mucous secretion clearance?
Key Findings
Relevant clinical effectiveness data were sparse. Mucous expectoration, mucous viscosity, and oxygenation tended to improve with acetylcysteine (NAC) compared with isotonic saline (IS), however the between-group differences were either not statistically significant or statistical significance was not reported.
For patients with chronic obstructive pulmonary disease or chronic bronchitis, or hospitalized patients with acute lung disease, findings were variable with respect to adverse events for treatment with NAC compared with placebo, and definitive conclusions were not possible. Other safety-related outcomes for the comparison of NAC versus placebo, such as hospitalization, atelectasis, and mortality, were sparsely reported and results were variable. Similarly, evidence for the safety of NAC compared to IS was sparse and definite conclusions were not possible.
No relevant evidence regarding the comparative clinical effectiveness of nebulized NAC versus oral NAC for patients requiring mucous secretion clearance were identified.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including MEDLINE, Embase, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were acetylcysteine and mucus or mucous secretions. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English-language documents published between January 1, 2014 and May 17, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Studies were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2014. Studies which included a mixed population (adult and pediatric) were excluded unless results were presented separately for adults. Studies already included in a selected systematic review were excluded.
Critical Appraisal of Individual Studies
The included systematic reviews were critically appraised by one reviewer using AMSTAR 2,8 and the included randomized controlled trial (RCT) was critically appraised based on the Downs and Black checklist.9 Summary scores were not calculated for the included studies; rather, the strengths and limitations of each individual study were described narratively.
Summary of Evidence
Quantity of Research Available
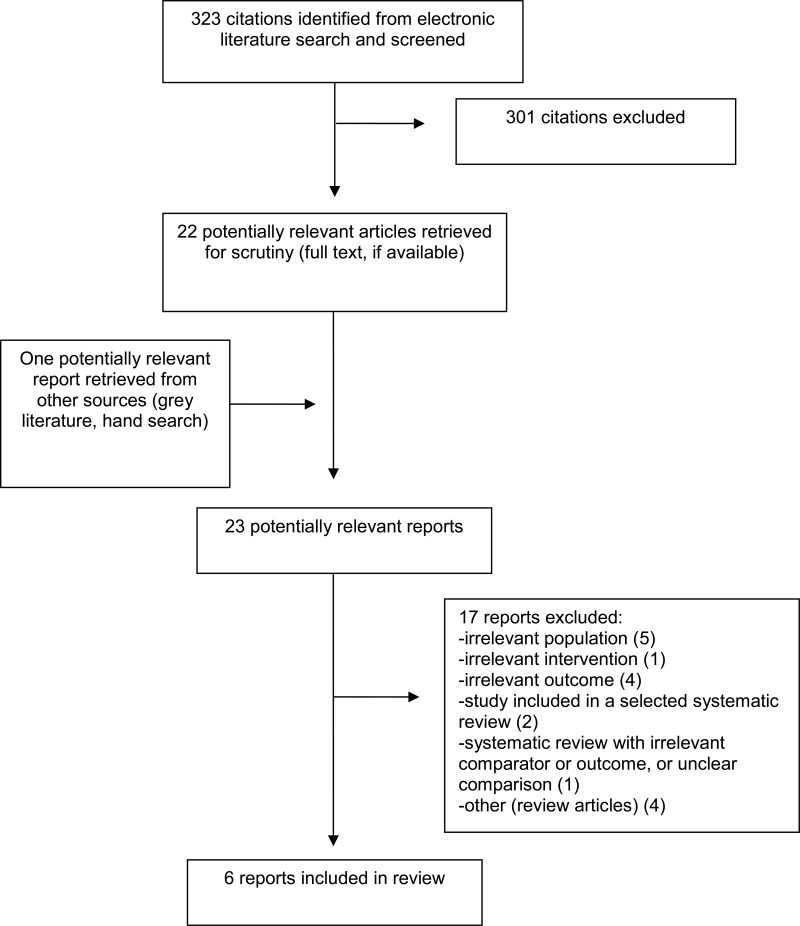
A total of 323 citations were identified in the literature search. Following screening of titles and abstracts, 301 citations were excluded and 22 potentially relevant reports from the electronic search were retrieved for full-text review. One potentially relevant publication was retrieved from the grey literature search for full text review. Of these potentially relevant articles, 17 publications were excluded for various reasons, and six publications met the inclusion criteria and were included in this report. These comprised five systematic reviews,1,4–7 and one RCT,10. Appendix 1 presents the PRISMA11 flowchart of the study selection.
Summary of Study Characteristics
Study characteristics are summarized in the following sections and additional details are provided in Appendix 2, and .
Study Design
Five relevant systematic reviews1,4–7 were selected. One systematic review7 included two relevant RCTs with ventilated or post-operative patients. One systematic review4 included four relevant RCTs with hospitalized patients. Three systematic reviews1,5,6 included RCTs involving patients with COPD or chronic bronchitis, and the numbers of included RCTs that were relevant for the current report were 14 in one systematic review,1 11 in another,5 and four in the third systematic review.6 It should be noted that there was overlap in the RCTs included in these three systematic reviews (Appendix 5).
The single included primary study10 was a single-centre, double-blind RCT involving hospitalized patients.
Country of Origin
One systematic review,1 published in 2019, was from the UK and included RCTs from Europe, China and India. A second systematic review7 published in 2019 was from Australia; countries of the included RCTs were not mentioned. One systematic review5 published in 2015 was from Italy; countries of the included RCTs were not mentioned. Another systematic review4 published in 2015 was from the US, and included RCTs from Australia, Denmark, Iran, and the US. The last systematic review,6 published in 2014, was from China; countries of the included RCTs were not mentioned.
The selected RCT,10 published in 2016, was from Turkey.10
Population
Three systematic reviews1,5,6 included adult patients (majority of patients age > 50 years) with COPD or chronic bronchitis; the total number of patients in the systematic reviews ranged between 516 and 3,882; and the proportion of males in the included individual RCTs ranged between 43% and 93%.
Two systematic reviews4,7 included hospitalized adult patients with acute lung disease and the total number of patients was 51 in one systematic review7 and 200 in the second systematic review.4. Of these, one systematic review7 included adult patients, but the mean age or the proportion of males were not reported. In the second systematic review4,4 the mean age ranged from 49 years to 74 years in the three included RCTs and was not reported in the last included RCT.
The selected RCT10 included 38 hospitalized patients; the mean age was 69 years and the proportion of males was 92%.
Interventions and Comparators
In four systematic reviews,1,4–6 NAC was compared with placebo. In three systematic reviews,1,5,6 oral NAC was used and in one systematic review7 inhaled NAC was used.. In one systematic review7 NAC was compared with isotonic saline (IS) and also NAC plus IS was compared with IS alone; both agents were inhaled.
In the selected RCT10 oral NAC was compared with placebo.
Outcomes
Outcomes reported included mucus characteristics,7 mucous expectoration,7 oxygenation,7 adverse events,1,4–7 atelectasis,4 hospitalization,1,4,10 and mortality.1,7
Summary of Critical Appraisal
Critical appraisal of the included studies is summarized below and details are presented in Appendix 3, and .
Overall the systematic reviews were well conducted. In all five systematic reviews1,4–7 the objective was clearly stated, a comprehensive literature search was conducted, article selection was described, a list of included studies was presented, study characteristics were described, and quality assessment of the studies were conducted and were found to be of variable quality. A list of excluded studies was presented in three systematic reviews1,4,7 and not presented in two systematic reviews.5,6 Article selection was done in duplicate in four systematic reviews,1,4,6,7 and not in one systematic review.5 Data extraction was done in duplicate in two systematic reviews, 1,6 and was unclear in three systematic reviews.4,5,7 In three systematic reviews1,5,6 meta-analyses were conducted, and in two systematic reviews4,7 it was not feasible to conduct a meta-analysis. In three systematic reviews1,6,7 it was reported that the authors had no conflicts of interest, in one systematic review5 conflicts of interest were declared and a few of the authors were associated with industry, and in one systematic review4 conflicts of interest were not reported.
In the selected RCT,10 the objective and inclusion and exclusion criteria were presented; the patient characteristics, intervention, and outcomes were described; and a sample size calculation was conducted, however the appropriate number of patients could not be enrolled during the study period. It was a randomized study, but the randomization method was not described. The intervention and control were identical in appearance (both capsules). It was reported that all parties involved with the RCT were blinded to the study medication. Five percent of patients in the NAC group and 14% in the placebo group discontinued within the first three days of the trial, due to worsening conditions, and were excluded from the analysis, The imbalance in the discontinuation rates could impact results, however the direction of impact is unclear. Discontinuation was < 15% in both groups, and the associated reasons were reported. Intention-to-treat analysis was not conducted. It was reported that the authors had no conflicts of interest.
Summary of Findings
Relevant study findings are summarized below and a table of the main study findings and authors’ conclusions are presented in Appendix 4, and . Of note, for studies comparing NAC with placebo, only the safety outcomes (not the effectiveness outcomes) were relevant of the current report and are presented here, as indicated in .
Clinical Effectiveness of Acetylcysteine (NAC)
One relevant systematic review,7 was identified regarding the clinical effectiveness of NAC compared with IS, in hospitalized patients with acute lung conditions (ventilated or post-operative). Relevant study findings are summarized and a table of the main study findings and authors’ conclusions are presented in Appendix 4, and .
Mucous expectoration
One RCT included in the selected systematic review7 showed that ease of mucous expectoration tended to be better with NAC compared to IS (numerically better on the visual analog scale 10) in post-operative patients, however the statistical significance of the between group difference was not stated.
Mucous characteristics
One RCT included in the selected systematic review7 reported that mucous viscosity improved with NAC but not with IS in post-operative patients, however the statistical significance of the between-group difference was not stated. The second RCT included in the selected systematic review7 reported that neither NAC nor IS lowered mucous density in ventilated patients.
Oxygenation
One RCT included in the selected systematic review7 reported that oxygenation (peripheral capillary oxygen saturation [SpO2] level) improved with NAC but not with IS, in post-operative patients, however the statistical significance of the between-group difference was not stated. The second RCT included in the selected systematic review7 reported that oxygenation improved with NAC and there was no change with IS, in ventilated patients.
Safety of Acetylcysteine (NAC)
Safety-related outcomes were available in all six included publications,1,4–7,10 however, the types of outcomes reported varied. Adverse events, were reported in five publications,1,4–7 atelectasis was reported in one publication,4 hospitalization was reported in three publications,1,4,10 and mortality was reported in two publications.1,7
Atelectasis
In the selected systematic review4 that included hospitalized patients, one included RCT found that fewer patients developed atelectasis with NAC compared with IS (however, the between-group difference was non-significant), and a second included RCT found that there was no significant between-group difference in atelectasis with NAC compared with placebo.
Adverse events
COPD and chronic bronchitis
Three systematic reviews1,5,6 that included patients with COPD and chronic bronchitis compared NAC with placebo and reported on adverse events; findings were variable and inconsistent. In one systematic review1 the odds ratios for adverse events in the individual included RCTs ranged from 0.36 to 2.05 and in majority of the RCTs the between-group differences were not statistically significant. In the second systematic review5 the relative risk (RR) of adverse events with NAC compared to placebo was 0.94, and 95% confidence interval (CI) was 0.88 to 0.99. In the third systematic review,6 the RR of adverse events was 1.30 (95% CI, 0.71 to 2.39); the between-group difference was statistically not significant.
Hospitalized patients
In one systematic review7 there were no adverse events reported with either NAC or IS, in post-operative or ventilated patients. One systematic review4 that included hospitalized patients, reported nausea in 10% of patients with NAC and 5% of the patients with placebo in one included RCT, and no adverse events during the study period in another included RCT. The single included primary study reported that 5% of patients in the NAC group and 14% in the placebo group discontinued within 3 days, due to worsening conditions and were excluded from the analysis; statistical significance of the difference in proportions was not presented.
Hospitalization
In one systematic review4 that included hospitalized patients, the median hospital stay was 6.0 days in the NAC group and 5.5 days in the placebo group (statistical significance of the findings was not reported). In the single included primary study,10 that included hospitalized patients, the length of hospital stay was 10.5 days in the NAC group and 9.8 in the placebo group; the between-group difference was not statistically significant. Also, in this RCT, there were no significant between-group differences for the number of hospital admissions or time to admission during the six month follow-up.
Mortality
In one systematic review1 that included patients with COPD or chronic bronchitis, the ORs for death for NAC compared with placebo in the individual included RCTs ranged from 0.13 to 3.24, and the between-group differences were not statistically significant.
In one systematic review7 that included hospitalized patients with acute lung conditions, the in-hospital mortality rate was 50% in the NAC group, and 35% in the IS group (data from one included RCT); statistical significance of the findings was not reported.
Clinical Effectiveness of Nebulized versus Oral Acetylcysteine (NAC)
No relevant evidence regarding the comparative clinical effectiveness of nebulized NAC versus oral NAC for patients requiring mucous secretion clearance were identified; therefore, no summary can be provided.
Limitations
There was considerable overlap in the RCTs included in three systematic reviews,1,5,6 hence findings are not exclusive, i.e. some of the same RCTs were used in assessing outcomes in these systematic reviews (Appendix 5).
There was a limited amount of evidence on the effectiveness of NAC compared with IS. No studies comparing NAC with pharmacologic agents (dornase alpha, guaifenesin, hypertonic saline or inhaled mannitol) or non-drug measures were identified.
Evidence on safety was available in terms of adverse events, atelectasis, hospitalization, and mortality, and not all of these outcomes were assessed in all of the included studies. One systematic review,{Poole, 2019 #331) though well conducted, reported summary estimates for several mucolytic agents taken together, hence only estimates from the individual studies on NAC could be incorporated in this current report.
No studies comparing nebulized NAC with oral NAC were identified.
Findings need to be interpreted with caution given these limitations.
Conclusions and Implications for Decision or Policy Making
A total of six relevant publications were identified regarding the clinical effectiveness or safety of NAC for patients requiring mucous secretion clearance. These comprised three systematic reviews1,5,6 and one RCT10 comparing NAC with placebo, one systematic review4 comparing NAC with both placebo and IS, and one systematic review7 comparing NAC with IS.
Relevant clinical effectiveness data were sparse. In one systematic review7 that included hospitalized patients with acute lung disease, one included RCT reported that mucous expectoration, mucous viscosity, and oxygenation improved with NAC compared with IS, however the between-group differences were either not statistically significant or statistical significance was not reported.
In terms of safety, for patients with COPD or chronic bronchitis, or for hospitalized patients with acute lung disease, findings were variable with respect to adverse events for treatment with NAC compared with placebo, and definitive conclusions were not possible. Other safety-related outcomes for the comparison of NAC versus placebo, such as hospitalization, atelectasis, and mortality, were sparsely reported and results were variable; definitive conclusions were not possible. Similarly, evidence for the safety of NAC compared to IS was sparse and definite conclusions were not possible.
No relevant evidence regarding the comparative clinical effectiveness of nebulized NAC versus oral NAC for patients requiring mucous secretion clearance were identified.
Studies on idiopathic pulmonary fibrosis patients did not meet the inclusion criteria for the present report as idiopathic pulmonary fibrosis is generally associated with non-productive cough (i.e., dry; not bringing up mucus). However the findings from these studies may provide some useful insights regarding treatment with NAC in comparison to placebo, and so are briefly discussed here. One systematic review12 showed that in terms of risk of serious adverse events or mortality, there were no statistically significant between-group differences for NAC compared to placebo. One RCT13 comparing NAC with placebo, reported numerically similar proportions of life-threatening adverse events (2% in each group), moderate adverse events (42% in NAC, 45% in placebo), and serious adverse events (5% in NAC, 3% in placebo); between-group differences were not tested statistically. One case-control study14 compared pirferindone plus NAC with pirferindone alone, and reported that no adverse events were attributed to NAC.
High-quality studies are needed to definitively determine the clinical effectiveness and safety of NAC. Also studies comparing NAC with other active pharmacologic treatments or non-drug treatments are warranted.
References
- 1.
Poole
P, Sathananthan
K, Fortescue
R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;5:Cd001287. [
PMC free article: PMC6527426] [
PubMed: 31107966]
- 2.
- 3.
- 4.
Sathe
NA, Krishnaswami
S, Andrews
J, Ficzere
C, McPheeters ML. Pharmacologic Agents That Promote Airway Clearance in Hospitalized Subjects: A Systematic Review.
Respir Care. 2015;60(7):1061–1070. [
PubMed: 25944943]
- 5.
- 6.
Shen. Effect of High/Low Dose N-Acetylcysteine on Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis.
COPD. 2014;11:351–358. [
PubMed: 24378052]
- 7.
Tarrant
BJ, Maitre
CL, Romero
L, Mucoactive agents for adults with acute lung conditions: A systematic review.
Heart Lung. 2019;48(2):141–147. [
PubMed: 30409442]
- 8.
- 9.
- 10.
Ayfer Aytemur
Z, Baysak
A, Ozdemir
O, Kose
T, Sayiner
A.N-acetylcysteine in patients with COPD exacerbations associated with increased sputum.
Wien Klin Wochenschr. 2015;127(7–8):256–261. [
PubMed: 25595117]
- 11.
Liberati
A, Altman
DG, Tetzlaff
J, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 12.
Rogliani
P, Calzetta
L, Cavalli
F, Matera
MG, Cazzola
M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis.
Pulm Pharmacol Ther. 2016;40:95–103. [
PubMed: 27481628]
- 13.
Behr
J, Bendstrup
E, Crestani
B, Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: A randomised, double-blind, placebo-controlled, phase 2 trial.
Lancet Respir Med. 2016;4(6):445–453. [
PubMed: 27161257]
- 14.
Sakamoto
S, Muramatsu
Y, Satoh
K, Effectiveness of combined therapy with pirfenidone and inhaled N-acetylcysteine for advanced idiopathic pulmonary fibrosis: A case-control study.
Respirology. 2015;20(3):445–452. [
PubMed: 25639750]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparators) | Clinical Outcomes,a Length of Follow-Up |
|---|
| Poole,1 2019, UK | SR included 14 relevant RCTs published between 1980 and 2014 (RCTs were: 3 Chinese, 2 European, 1 German, 1 Netherlands, 2 Indian, 2 Italian, 1 Swedish, 2 UK)
Setting: Outpatient or general practice
(This SR had a broad focus and included placebo-controlled RCTs; only RCTs relevant for this current report are included here) | Patients with COPD or chronic bronchitis
N = 3,882 (primary study size ranged from 59 to 990)
Age (mean) (years): 60 to 71 in 12 RCTs; age >20 years in 1 RCT; and age >50 years in 60% patients in 1 RCT.
% Male: 59% to 93% in 13 RCTs, and not reported in 1 RCT | NAC versus placebo
NAC dose:
200mg bid (2 RCTs),
200 mgtid (2 RCTs),
300mg bid (1 RCTs),
600 mg daily (5 RCTs),
600 mg bid (3 RCTs),
600 mg tid (1 RCT), | Adverse effects, hospitalization, mortality.
Duration of primary studies: 3 months to 3 years.
(Note: a number of studies could not be included in the analysis as the number of adverse events exceeded the numbers included in the treatment groups) |
| Tarrant,7 2019, Australia | SR included 2 relevant RCTs published between 1970 and 1992 (country: NR)
Setting: hospital
(This SR had a broad focus [mucoactive agents] and included several comparators; only RCTs relevant for this current report are included here) | Adults with acute lung condition (ventilated patients or postoperative patients)
N = 51 (40 and 11 in the two primary studies)
Age (years): NR
% Male: NR | NAC versus IS (both nebulized)
NAC (20%) 4 mL versus IS (0.9%) 4 mL in RCT in postoperative patients.
(NAC [20%], 2 mL + IS, 8 mL) versus(IS [0.9%], 10 mL) in RCT in ventilated patients | Mucus characteristics, mucous expectoration, oxygenation
Adverse events, mortality
Durations of primary studies: unclear |
| Cazzola,5 2015, Italy | SR included 11 relevant RCTs published between 1976 and 2014 (country: NR)
Setting: NR
(This SR included placebo-controlled RCTs; only RCTs relevant for this current report are included here) | Patients with COPD or chronic bronchitis
N = 2,828 (primary study size [patients who completed study] ranged from 45 to 964)
Age (mean) (years):51 to 71 in 10 RCTs; and NR in 1 RCT
% Male: 43% to 93% | NAC versus placebo
NAC daily dose:
260 mg in 1 RCT,
400 mg in 2 RCTs
600 mg in 5 RCTs,
1,200 mg in 3 RCTs, | Adverse events
Durations of primary studies: 4 months to 36 months |
| Sathe,4 2015, US | SR included 4 relevant studies (3 RCTs and1 non-randomized study) published between 1966 and 2010 (Country: one RCT each in Australia Denmark, Iran and the US)
Setting: hospital
(This SR had a broad focus [assessed various pharmacologic agents that promote airway clearance]; only RCTs relevant for this current report are included here) | Hospitalized patients (with COPD exacerbations, asthma exacerbations, or postoperative)
N = 269 (=50+50+129+40) enrolled, and 200 (=50+50+60+40) final number of patients
Age (years) (mean): 49, 53 and 74 for 3 studies; and NR for 1 study
% Male: NR | NAC versus placebo (3 RCTs),
Oral NAC 600 mg, bid; versus placebo (2 RCTs).
Oral NAC 1,200 mg on day before surgery, and oral or intravenous NAC 200 mg, tid for 6 days or until discharge (1 RCT)
------------------------------
NAC versus saline (1 study)
NAC 10% solution, 2 ml every 2 h for 10 doses after anesthesia recovery and physiologic saline 2 mL every 2 h | For NAC versus placebo studies: adverse events, hospital stay, atelectasis
NAC versus saline study:
atelectasis, nausea |
| Shen,6 2015, China | SR included 4 relevant RCTs published between 1985 and 2013 (country: NR)
Setting: NR
(This SR included placebo controlled RCTs; only RCTs relevant for this current report are included here) | Patients with COPD or chronic bronchitis
N = 516 (primary study size ranged from 91 to 169)
Age (mean) (years):51 to 71 in 10 RCTs; and NR in 1 RCT
% Male: 43% to 93% | NAC versus placebo
NAC dose:
200 mg tid (1 RCT),
300 mg bid (1 RCT),
600 mg daily (1 RCT),
600 mg bid (1 RCT), | Adverse events (gastrointestinal)
Durations of primary studies: 5 months to 1 year |
bid = two times daily; COPD = chronic obstructive pulmonary disease; IS = isotonic saline; NAC = N-acetylcysteine; neb = nebulizer; NR = not reported; RCT = randomized controlled trial; SR = systematic review; tid = three times daily.
- a
Only outcomes relevant for the current report are included here.
Table 3Characteristics of Included Primary Clinical Study
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparators) | Clinical Outcomes,a Length of Follow-Up |
|---|
| Randomized controlled trials |
|---|
| Ayfer Aytemur,10 2015, Turkey | RCT, double blinded, single center Setting: single center | Patients with COPD who were hospitalized for their current exacerbation
N = 42 (38 were analyzed; 19 in each group)
Age (mean) (years):
68.6 in NAC group and 69.4 in placebo group
% Male: 89 in NAC group and 95% in placebo group
Duration of COPD (years): 19 ± 14.9 in NAC group and 11.4 ± 7.5 in placebo group
| NAC versus placebo NAC capsules 200 mg tid for 30 days | Hospitalization Follow-up = 6m |
COPD = chronic obstructive pulmonary disease; m = month; NAC = N acetylcysteine; RCT = randomized controlled trial; tid = three times daily.
- a
Only outcomes relevant for the current report are included here.
Appendix 3. Critical Appraisal of Included Publications
Table 4Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR 28
View in own window
| Strengths | Limitations |
|---|
| Poole,1 2019, UK |
|---|
The objective was clearly stated Multiple databases (MEDLINE, EMBASE, Airways Group Trials Register, PsychlNFO, CINAHL) were searched up to April 2019 (a previous version of the review included searches up to July 2014 and this current version included also additional search between July 2014 to April 2019). In addition proceedings of major respiratory conferences, and reference list of included studies and reviews, were searched. Study selection was described and a flow chart was presented A list of included studies was provided A list of excluded studies was provided Article selection was done independently by two reviewers Data were extracted by two reviewers. This Cochrane review was an update from a previous Cochrane review and the extracted data were double checked against the original publication. Quality assessment was conducted using the Cochrane risk of bias tool; the studies were of variable quality Characteristics of the included studies were presented Meta-analysis was conducted. However as all mucolytic agents were included in the meta-analysis, pooled estimates for NAC alone could not be presented; instead, individual estimates were considered. Publication bias was explored using Funnel plots when feasible (i.e., > 10 studies were available). The quality of the studies was variable. It was mentioned the authors had no known conflicts of interest
|
|
| Tarrant,7 2019, Australia |
|---|
The objective was clearly stated Multiple databases (Medline, Embase, CINAHL and CENTRAL) up to January 2018. In addition grey literature was searched. Study selection was described and a flow chart was presented A list of included studies was provided A list of excluded studies was provided Article selection was done by two reviewers Quality assessment was conducted using the Cochrane risk of bias tool (7 items). For the 7 items, risk of bias was low for 4 items, unclear for 2 items, and high for 2 items for both of the two relevant RCTs. Risk of bias was low for some items and unclear or high for some items. Characteristics of the included studies were presented, however lacked some details (e.g., age of the population, duration of the study) Narrative synthesis was done (the authors mentioned that if meta-analysis was not possible, a narrative synthesis would be done) It was mentioned the authors had no known conflicts of interest
|
|
| Cazzola,5 2015, Italy |
|---|
The objective was clearly stated The search was performed on PubMed and Google scholar up to July 2014. Also relevant reviews and meta-analysis were examined to identify studies. Study selection was described and a flow chart was presented A list of included studies was provided Quality assessment was conducted using the Jadad score (scale: 1 to 5, with higher scores indicating better quality). Scores ranged from 1 to 4, with 55% of the studies having a score of 4 Characteristics of the included studies were presented Meta-analysis was conducted
|
A list of excluded studies was not provided Unclear if article selection was conducted in duplicate Unclear if data extraction was done in duplicate Publication bias does not appear to have been examined Declarations were provided by two of the seven authors, and both received fees (not related to the current review) from industry
|
| Sathe,4 2015, US |
|---|
The objective was clearly stated Multiple databases (MEDLINE, EMBASE) were searched from 1970 until July 2014. Also, reference lists of include studies and relevant narrative and systematic reviews and meta-analysis were searched. Study selection was described and a flow chart was presented A list of included studies was provided A list of excluded studies was provided Article selection was done independently by two reviewers Quality of the studies was assessed by independently by two reviewers, using the Cochrane Risk of Bias tool for RCTs and the Newcastle-Ottawa scale for non-randomized studies. The majority of the studies were judged to be of low quality. Characteristics of the included studies were presented
|
Unclear if data extraction was done in duplicate Meta-analysis was not conducted Publication bias does not appear to have been examined Conflicts of interest of the authors were not presented
|
| Shen,6 2014, China |
|---|
The objective was clearly stated Multiple databases (MEDLINE, EMBASE, Cochrane library) were searched until August 2013. Also, reference lists of include studies and relevant reviews were searched. Study selection was described and a flow chart was presented A list of included studies was provided Article selection was done independently by two reviewers Data was extracted and rechecked independently by two reviewers Quality of the studies was assessed by independently by two reviewers using the Cochrane Allocation Concealment Scale and Jadad score (scale 1 to 5, with higher scores indicating better quality). Jadad score ranged from 2 to 4 with 75% of the studies having score of 4. Characteristics of the included studies were presented Meta-analysis was conducted The authors mentioned that there were no conflicts of interest
|
|
NAC = acetylcysteine; RCTs = randomized controlled trials.
Table 5Strengths and Limitations of Clinical Study using Downs and Black checklist9
View in own window
| Strengths | Limitations |
|---|
| Ayfer Aytemur,10 2015, Turkey |
|---|
The objective was clearly stated The inclusion and exclusion criteria were stated Patient characteristics, intervention and outcomes were described Randomized study but randomization procedure was not described Double-blinded – all parties were blinded to the study medication the patients received Sample size calculation was conducted, however, the appropriate number of patients could not be reached during the study period. Discontinuation and the associated reasons were reported; 5% in the NAC group and 14% in the placebo group were excluded as they had worsened and had to be admitted to the ICU and intubated. The authors mentioned that there were no conflicts of interest P values were reported
|
|
ICU = intensive care unit; NAC = acetylcysteine.
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 6Summary of Findings Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findingsa | Authors’ Conclusion |
|---|
| Poole,1 2019, UK |
|---|
Comparison of treatment with NAC (a mucolytic agent) versus placebo, with respect to safety for patients with COPD or chronic bronchitis (from SR). Adverse effects were reported in 12 RCTs. In 2 RCTs the ORs were 0.36 and 0.54 and the between-group differences were statistically significant, favoring NAC. However, in 9 RCTs, the ORs ranged from 0.48 to 2.05, and the between-group differences were statistically non-significant. In 1 RCT there were zero adverse events reported in both groups. Hospitalization during study period was reported in 4 RCTs. In 1 RCT the OR was 0.32 and the between-group difference was statistically significant favoring NAC; however in 3 RCTs, ORs ranged from 0.61 to 0.91 and the between-group differences were statistically non-significant. Death during study period was reported in 8 RCTs. In 6 RCTs, ORs ranged from 0.13 to 3.24 and the between-group differences were statistically nonsignificant. In 2 RCTs, there were zero adverse events reported in both groups. | “There was no clear difference between mucolytics and placebo for mortality, but the confidence interval is too wide to confirm that treatment has no effect on mortality” (p. 2) “People taking mucolytics did not experience more unwanted side effects than those taking placebo.” (P-3) |
| Tarrant,7 2019, Australia |
|---|
Comparison of treatment with nebulized NAC versus nebulized isotonic saline (IS) with respect to efficacy and safety for patients with acute lung conditions (from SR with 2 relevant RCTs) Mucus characteristics One RCT compared NAC with IS, and showed that after two days of use of either NAC or IS following thoracic or abdominal surgery, with NAC the mean mucus weight (g) increased (mean ± standard deviation [SD] from 2.65 ± 3.47 to 7.50 ± 6.29; P = 0001) and with IS there was little change (mean ± SD: from 3.45 ± 2.16to 3.55 ± 2.99; not statistically significant); and mucus viscosity improved with NAC but not with IS, however the significance of the between-group difference was not stated in either instance. One RCT compared NAC with IS, and showed that during invasive ventilation neither NAC nor IS lowered mucus density after three doses over 24 hours. Mucous expectoration One RCT compared NAC with IS, and showed that after two days of use of either NAC or IS following thoracotomy or laparotomy, with NAC the ease of mucous expectoration improved (3.75 cm) and there was little improvement with IS (0.27 cm), using visual analog scale of 10, however the significance of the between-group difference was not stated Oxygenation One RCT showed that, post thoracic or abdominal surgery, oxygenation (SpO2 [%]) improved with NAC (from 91.6 ± 3.75 to 93.96 ± 2.67) and there was no change with IS (93.08 ± 3.23 to 93.35 ± 3.64), but the significance of the between group difference was not reported. One RCT showed that, during invasive ventilation, oxygenation (SpO2) improved with NAC (from 93.8 ± 2.7 to 95.1 ± 2.6) and there was no change with IS (94.0 ± 2.2 to 93.9 ± 2.5), however the between-group difference was not statistically significant (P= 0.30) Adverse events There were no AEs reported with either NAC or IS, in post-operative or ventilated patients. Mortality In one RCT, for the ventilated patients who had a diagnosis of pneumonia or sepsis, the in-hospital mortality rate was 50% in the NAC group and 35% in the IS group; statistical significance of the findings was not reported. | The authors reported that for post-surgery patients with acute lung conditions, mucous weight, viscosity, and expectoration; and oxygenation improved with NAC and there was little change with IS, however the significance of between-group differences were not reported. Also, for ventilated patients with acute lung conditions there was no improvement in mucous density with either NAC or IS; there was some improvement in oxygenation with NAC and little change with IS, however the between-group difference was not statistically significant. There were no AEs reported with either NAC or IS in post-operative or ventilated patients. |
| Cazzola,5 2015, Italy |
|---|
| Comparison of treatment with NAC (a mucolytic agent) versus placebo, with respect to safety for patients with COPD or chronic bronchitis (from SR). | “NAC was well tolerated and the risk of adverse reactions was not dose dependent”, (p. 451) |
| NAC dose | No. of RCTs | RR (95% Cl) | Heterogeneity, I2 (%) |
|---|
| All (260 mg to 1,200 mg daily) | 11 | 0.94 (0.88 to 0.99) | 5 |
| Low (260 mg to 600 mg daily) | 8 | 0.93 (0.89 to 0.97) | 0 |
| High (1,200 mg daily) | 3 | 1.11 (0.89 to 1.39) | 0 |
| Adverse events reported included gastrointestinal disorders, respiratory disorders, and other disorders (such as pyrosis, joint and muscle pain, and dizziness) |
| Sathe,4 2015, US |
|---|
Comparison of treatment with NAC (a mucoactive agent) versus placebo, or saline with respect to adverse events for hospitalized patients requiring airway clearance (from SR). Atelectasis In one RCT, 4 of 20 patients in the NAC group and 9 of 20 patients in the saline group developed atelectasis, however the between-group difference was reported as not significant. In one RCT there was no significant difference in atelectasis between the NAC group and the placebo group. Adverse events 2 patients in the NAC group and 1 patient in the placebo group had nausea; both groups comprised 20 patients each (1 RCT) AE not mentioned in 1 RCT (NAC vs. placebo) No adverse effects were reported during the study period (1 RCT: NAC vs. placebo) AE not mentioned in 1 RCT (NAC versus saline) Hospital stay Hospital stay (median) was 6.0 days in the NAC group and 5.5 days in the placebo group (P value not reported) (from 1 RCT) | For hospitalized patients requiring airway clearance Sathe et al. mentioned that “Further research with clearly characterized populations and interventions is needed to understand the potential benefits and adverse effects of mucoactive agents.” (p. 1061) |
| Shen,6 2014, China |
|---|
| Comparison of treatment with NAC (a mucolytic agent) versus placebo, with respect to adverse events (Gl disorders) for patients with COPD or chronic bronchitis (from SR). | “Gl disorders including diarrhea, reflux esophagitis, and gastric complications were reported in some studies, but NAC did not significantly increase the risk of such adverse reactions” (p. 355) |
| No. of RCTs | RR (95% Cl) | Heterogeneity, I2 (%) |
|---|
| 4 | 1.30(0.71 to 2.39) | 0 |
Cl = confidence interval; COPD = chronic obstructive pulmonary disease; Gl = disorder; IS = isotonic saline; NAC = N-acetylcysteine; OR = odds ratio; RCT = randomized controlled trial; RD = risk difference; RR = relative risk; SpO2 = peripheral capillary oxygen saturation; SR = systematic review.
- a
Only outcomes of interest for the current report are included here (i.e., clinical effectiveness outcomes with NAC compared to placebo or no treatment are not reported here).
Table 7Summary of Findings of Included Primary Clinical Study
View in own window
| Main Study Findingsa | Authors’ Conclusion |
|---|
| Randomized controlled trial |
|---|
| Ayfer Aytemur,10 2015, Turkey |
|---|
| Comparison of treatment with NAC versus placebo, with respect to hospitalization for COPD patients who were hospitalized for their current exacerbation (RCT) | “In conclusion, we found that NAC given at a daily dose of 600 mg to patients with COPD exacerbations and with a high volume of sputum production did not affect symptoms, pulmonary function, length of hospital stay, and exacerbation rate during the follow-up period.” (p.260) |
| Outcome | Effect | P value |
|---|
| NAC | Placebo |
|---|
| Length of hospital stay (d) | 10.5 ± 3.8 | 9.8 ± 3.0 | 0.52 |
| Number of hospital admissions during 6 m FU | 0.9 ± 1.1 | 0.6 ± 0.9 | 0.42 |
| Time (d) to hospital admission during 6 m FU | 45.6 ± 67.2 | 24.6 ± 41.5 | 0.37 |
| Unclear if effect was expressed as mean and standard deviation |
d = day; m = month; NAC = N-acetylcysteine.
- a
Only outcomes of interest for the current report are included here (i.e., clinical effectiveness outcomes with NAC compared to placebo or no treatment are not reported here.
Appendix 5. Overlap between Included Systematic Reviews
Table 8Primary Study Overlap between Included Systematic Reviews
View in own window
| Primary Studya Citation | Systematic Review Citation |
|---|
| Poole,1 2019 | Cazzola,5 2015 | Shen,6 2015 |
|---|
| Babolini, 1980 | × | × | |
| Bachh, 2007 | × | × | |
| Boman, 1983 | × | × | |
| Decramer, 2005 | × | × | × |
| Grassi, 1976 | × | × | × |
| Hansen, 1994 | × | × | × |
| Jackson, 1984 | × | | |
| Johnson, 2016 | × | | |
| McGavin, 1985 | × | × | × |
| Meister, 1986 | × | | |
| Nowak, 1999 | × | | |
| Pela, 1999 | × | × | × |
| Rasmussen, 1988 | × | × | × |
| Schermer, 2009 | × | × | × |
| Tse, 2013 | × | × | × |
| Xu, 2014 | × | | |
| Zheng, 2014 | × | × | |
× indicates studies that were included in the systematic review.
- a
Primary studies which reported on outcomes of relevance to the current report.
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Acetylcysteine for Patients Requiring Mucous Secretion Clearance: A Review of Clinical Effectiveness and Safety. Ottawa: CADTH; 2019 Jun. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.