This publication is in the public domain and is therefore without copyright. All text from this work may be reprinted freely. Use of these materials should be acknowledged.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
The ESP Coordinating Center (ESP CC) is responding to a request from the Partnered Evidence-Based Policy Resource Center (PEPReC) for an evidence brief on the relation between receipt of preventive dental care and chronic disease-related benefits, harms, and costs. Findings from this evidence brief will be used to inform implementation and evaluation of the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits demonstration program.
PREFACE
The VA Evidence Synthesis Program (ESP) was established in 2007 to provide timely and accurate syntheses of targeted healthcare topics of importance to clinicians, managers, and policymakers as they work to improve the health and healthcare of Veterans. These reports help:
- Develop clinical policies informed by evidence;
- Implement effective services to improve patient outcomes and to support VA clinical practice guidelines and performance measures; and
- Set the direction for future research to address gaps in clinical knowledge.
The program comprises three ESP Centers across the US and a Coordinating Center located in Portland, Oregon. Center Directors are VA clinicians and recognized leaders in the field of evidence synthesis with close ties to the AHRQ Evidence-based Practice Center Program and Cochrane. The Coordinating Center was created to manage program operations, ensure methodological consistency and quality of products, and interface with stakeholders. To ensure responsiveness to the needs of decision-makers, the program is governed by a Steering Committee composed of health system leadership and researchers. The program solicits nominations for review topics several times a year via the program website.
Comments on this evidence report are welcome and can be sent to Nicole Floyd, Deputy Director, ESP Coordinating Center at vog.av@dyolF.elociN.
Executive Summary
Background
The ESP Coordinating Center (ESP CC) is responding to a request from the Partnered Evidence-Based Policy Resource Center (PEPReC) for an evidence brief on the relation between receipt of preventive dental care and chronic disease-related benefits, harms, and costs. Findings from this evidence brief will be used to inform implementation and evaluation of the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits demonstration program.
Methods
To identify systematic reviews, we searched MEDLINE and CDSR up to October 2020. We also searched MEDLINE and CENTRAL up to December 2020 for primary studies that addressed gaps in systematic review evidence and studies published after included systematic reviews’ searches. We used prespecified criteria for study selection, data abstraction, and rating the quality and strength of the evidence. See our PROSPERO protocol for our full methods (Registration # CRD42020215625).
Key Findings
- For adults with chronic obstructive pulmonary disease (COPD), periodontal treatment may improve lung function and reduce the frequency of exacerbations at 1 and 2 years compared to no treatment based on 2 fair-quality controlled trials. Periodontal treatment may also contribute to lower annual medical costs based on 1 poor-quality retrospective cohort study.
- For adults with type 2 diabetes, periodontal treatment likely improves measures of chronic disease severity and inflammation (eg, HbA1c, fasting blood glucose, total cholesterol, CRP) with only minor adverse events in the short term (3-4 months) based on several moderate- and high-quality systematic reviews and newly published RCTs. These improvements do not appear to persist beyond 6 months, however. Findings are unclear on the relation between periodontal treatment and diabetes-related complications and costs.
- For adults with cardiovascular disease, periodontal treatment likely improves measures of inflammation (eg, TNF-α, IL-6 and CRP) at 3 months based on 1 moderate- and 1 high-quality systematic review; longer-term outcomes have not been evaluated. Findings are unclear on the relation between periodontal treatment and cardiovascular-related complications and costs.
- There is limited available evidence on the effect of periodontal treatment among adults with cerebrovascular disease. Existing studies are unclear on the relation of periodontal treatment to complications and costs, similar to findings for diabetes and cardiovascular disease.
Poor dental health in the form of periodontal disease (a gum infection typically caused by poor oral hygiene) is a widespread issue in the United States. Nearly half of US adults aged 30 and older have periodontal disease, and prevalence is highest among older adults (70% of those 65 or older), men (56%), smokers (64%), and those below the federal poverty level (65%). In the last 2 decades, growing evidence has suggested periodontal disease is associated with chronic diseases such as heart disease, lung disease, stroke, and type 2 diabetes. The pathway between periodontal disease and chronic diseases is not well understood, but periodontal disease may trigger an inflammatory response in the body that leads to high blood pressure, vascular inflammation, and/or atherosclerosis; these processes then contribute to the etiology and severity of certain chronic diseases. Bacteria in the oral cavity may also be inhaled into the lower respiratory tract, leading to exacerbations of chronic obstructive pulmonary disease (COPD).
Detection and initial treatment of dental problems, including periodontal disease, is commonly implemented through the provision of preventive dental services such as routine exams, cleanings, and education on oral hygiene. Treatment of more advanced forms of periodontal disease typically consists of scaling and root planing, with or without the use of antibiotics. Currently, few Veterans receive these services: only 8% of Veterans have a dental issue that is service-connected, or meet other criteria required to receive dental care through the Department of Veterans Affairs (VA). In 2019, the VA initiated the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits program to increase Veterans’ access to community-based, pro bono or discounted dental service providers. Although the intended purpose of the program is to reduce the use of VA emergency care for dental conditions by providing Veterans with access to routine dental care, the program may also improve Veterans’ chronic disease outcomes, given the link between periodontal disease treatment and chronic disease outcomes. The purpose of the current ESP review is to synthesize evidence on benefits and harms of detection and treatment of dental problems (specifically, periodontal disease) among those with type 2 diabetes, cardiovascular disease, cerebrovascular disease, or COPD. Evidence on chronic disease outcomes, health care utilization, and costs associated with periodontal treatment will be used to inform the implementation and evaluation of the VA Care Coordination for Dental Benefits program.
Using rapid review methods, we prioritized evidence from the most recent and relevant systematic reviews (SRs) and only included primary studies when they addressed gaps in higher-level evidence and when they were published after the search dates of prioritized SRs. From 1,768 possible citations, we included 46 studies; from these, we prioritized 8 SRs and included 21 primary studies that addressed gaps or updated evidence from SRs. Highlighted findings from these 29 studies appears in Table 1.
For those with COPD, evidence from 3 controlled trials suggests that periodontal treatment improves certain chronic disease outcomes. One fair-quality RCT and 1 fair-quality non-randomized, controlled trial indicate periodontal treatment may improve lung function and reduce the frequency of exacerbations at 1 and 2 years compared to no treatment. Another fair-quality RCT found no adverse events within a month of periodontal treatment. However, in this short study, periodontal treatment did not reduce the number of doctor’s visits after treatment, nor did it improve quality of life, self-assessment of health, or illness severity. An additional poor-quality retrospective cohort study indicated those with COPD who receive periodontal treatment have lower annual medical costs than those who do not receive treatment.
Results from moderate- and high-quality SRs suggest periodontal treatment improves some chronic disease and inflammatory indicators in the short term for people with type 2 diabetes (eg, HbA1c, fasting blood glucose, total cholesterol, CRP) or cardiovascular disease (eg, TNF-α, IL-6, CRP, LDL cholesterol) with only minor adverse events. However, improvements do not seem to last beyond 6 months. The impact of periodontal treatment on chronic disease indicators for those with cerebrovascular disease is unclear.
Table 1
Highlighted Review Findings.
Evidence is also unclear on whether periodontal treatment reduces the risk of diabetes and cardiovascular disease-related complications. For those with type 2 diabetes, evidence from a single fair-quality retrospective cohort study indicates periodontal treatment may reduce the risk of heart failure compared to no treatment. Findings on stroke and myocardial infarction are unclear, with some studies finding lower risk among people with type 2 diabetes who receive periodontal treatment compared to no treatment and others finding no difference in risk between groups. For those with hypertension or atherosclerosis, evidence from a single, poor-quality retrospective cohort study indicates periodontal treatment is associated with a reduced risk of stroke. However, a self-controlled case series indicates that individuals’ risk for stroke and myocardial infarction actually increased after periodontal treatment when compared to baseline. It is possible the results of this study were influenced by patients discontinuing NSAIDS, blood thinners, or antiplatelet medications due to periodontal treatment, which could have caused an increase in cardiovascular risk. In this study, the risk of MI decreased over time, while for stroke, the pattern of resolution was unclear.
Findings on costs of periodontal treatment versus no treatment for people with type 2 diabetes, coronary artery disease (CAD), or cerebrovascular disease are similarly unclear, with some studies indicating periodontal treatment contributes to lower medical costs and other studies indicating periodontal treatment has no effect or contributes to higher health care costs. One poor-quality retrospective cohort study found those with congestive heart failure who undergo periodontal treatment have lower medical costs than those who do not receive treatment. In terms of health care utilization, 1 poor-quality retrospective cohort study reported periodontal treatment was associated with lower inpatient admissions for those with type 2 diabetes, CAD, or cerebrovascular disease, while another found no significant difference in health care utilization in those with type 2 diabetes who do and do not receive periodontal treatment.
There is some evidence that the balance of benefits and costs of periodontal treatment may differ by individual patient characteristics. For example, in 1 study that reported periodontal treatment was associated with higher medical costs compared to no treatment, authors noted that periodontal treatment was most cost-effective among those with higher HbA1c (who may have more to gain from periodontal treatment-associated improvements in HbA1c) and those who are older (who may have lower lifetime costs of periodontal treatment). Another found savings due to periodontal treatment were limited to those who did not initiate diabetes-related medications after diagnosis.
There are limitations to our rapid review methodology and of our included studies. We used rapid review methods to prioritize synthesis of the best available evidence, rather than all available evidence. These methods included: 1) synthesizing the most recent and relevant SRs, 2) conducting primary study searches to address gaps in the systematic review literature and to identify evidence published after SRs, and 3) having a single reviewer assess study eligibility, study quality, and strength of evidence with second reviewer checking. Common limitations of prioritized SRs included not searching for grey literature, having no publicly available protocol, and lacking discussion of individual studies’ risk of bias. Common limitations of included primary studies were lack of patient and provider blinding in RCTs, and, in controlled observational studies, poorly defined treatment and control groups and the presence of important differences between groups at baseline.
As we primarily found unclear results from studies with methodological limitations, there is a need for better-conducted studies on this topic. Future research should evaluate the effect of periodontal treatment on a broad range of clinically relevant outcomes (including quality of life, diabetes and cardiovascular disease-related complications, and potential harms) measured at shorter (3-6 months) and longer-term (1+ years) time points. VA researchers may additionally wish to use a hybrid effectiveness-implementation study design to evaluate clinically relevant questions around whether referral to dental care improves outcomes.
In conclusion, among people with COPD, periodontal treatment may improve lung function and reduce exacerbations at 1-2 years and reduce annual medical costs. Among people with diabetes or cardiovascular disease, periodontal treatment likely improves some measures of chronic disease severity and inflammation at 3-4 months, but benefits do not appear to persist beyond 6 months. Results are unclear on the relation between periodontal treatment and chronic disease outcomes for those with cerebrovascular disease. Results are also unclear on the relation between periodontal treatment, medical costs, and risk of chronic disease complications among those with diabetes, cardiovascular disease, or cerebrovascular disease.
INTRODUCTION
PURPOSE
The ESP Coordinating Center (ESP CC) is responding to a request from the Partnered Evidence-Based Policy Resource Center (PEPReC) for an evidence brief on the relation between receipt of preventive dental care and chronic disease-related benefits, harms, and costs. Findings from this evidence brief will be used to inform implementation and evaluation of the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits demonstration program.
BACKGROUND
Poor dental health in the form of periodontal disease is a widespread issue in the United States. Nearly half of US adults aged 30 and older have periodontal disease, and prevalence is highest among older adults (70% of those 65 or older), men (56%), smokers (64%), and those below the federal poverty level (65%).1 While periodontitis and related conditions such as tooth decay compromise oral health and oral quality of life, there is increasing evidence that oral health impacts non-oral chronic diseases such as heart disease, lung disease, stroke, and diabetes. Early evidence of this association was summarized in a 2000 report from United States Surgeon General,2 and in the decades since that report, research on the topic has accelerated.
Most research to date has examined the relation of periodontal disease with cardiovascular disease (CVD) and type 2 diabetes (T2D) outcomes. A 2012 systematic review (SR) by the American Heart Association concluded there is an association between periodontal disease and atherosclerotic vascular disease (ASVD) that is independent of known risk factors.3 In this SR, 18 out of 26 relevant studies reported poor periodontal status was associated with increased risk of ASVD-related outcomes (such as coronary heart disease [CHD], coronary artery disease [CAD], and mortality from ASVD and CHD causes). Eleven out of 14 relevant studies found poor periodontal status was linked with increased risk of stroke. Along the same lines, a 2013 SR by the European Federation of Periodontology and the American Academy of Periodontology found a “small body of evidence [that] supports significant, adverse effects of periodontal disease on glycaemic control, diabetes complications, and development of type 2 (and possibly gestational) diabetes.”4 Additionally, a 2006 SR5 and several subsequent observational studies6,7 have suggested there may be a relation between periodontal disease and respiratory diseases such as chronic obstructive pulmonary disease (COPD).
Periodontal disease (which includes gingivitis and periodontitis) is a gum infection that typically results from poor oral hygiene. Symptoms include bleeding or receding gums in mild cases and painful abscesses and loss of teeth in severe cases.8 Because periodontal disease triggers an inflammatory response in the body, it may contribute to worsening of diseases whose etiology or severity are in part driven by chronic inflammation.4,9 Importantly, however, this pathway is not fully understood and may differ by chronic disease. For instance, it is possible that periodontal disease may cause or worsen some chronic diseases, but it may also be the case that the conditions have 1 or more shared causes, such as smoking, alcohol misuse, overweight/obesity, or socioeconomic factors. For T2D in particular, there is considerable evidence that while periodontal disease may impact glycemic control, T2D itself likely worsens oral health (periodontal disease has been recently described as the “sixth complication of diabetes”).3,10,11
There are several plausible biological mechanisms that may explain the link between periodontal disease and chronic disease outcomes. One possibility is that periodontal disease-related inflammation causes the destruction of endothelial cells that line the walls of blood vessels and maintain normal blood pressure.9 High blood pressure is a risk factor for development of CVD and cerebrovascular diseases as well as adverse events such as myocardial infarction and stroke.12 A second potential pathway is that oral bacteria such as porphyromonas gingivalis directly invade arterial walls through the breakdown of gum tissue, causing vascular inflammation and atherosclerosis.9 For those with COPD, oral bacteria can be inhaled into the lower respiratory tract, which can eventually trigger COPD-related exacerbations.7 Finally, periodontal disease may trigger production of highly reactive chemical molecules that cause oxidative stress throughout the body.9 For people with T2D, oxidative stress may contribute to vascular complications such as retinopathy, nephropathy, and cardiomyopathy.13
Despite growing evidence linking periodontal disease to chronic disease outcomes, it is unclear whether interventions that have been shown to successfully treat periodontal disease can also lead to clinically meaningful improvement in CVD, T2D, or other chronic disease outcomes. There is a pressing need to address this question given the high comorbidity of chronic diseases among those with, or at risk for, periodontal disease. For example, in a recent case-control study of 1,199 people with periodontal disease, 19% had comorbid hypertension, 9.7% had comorbid endocrine disorders, and 8.5% had comorbid pulmonary disorders.14 Chronic conditions are especially prevalent among Veterans: it has been estimated that 43% of Veterans have hypertension, 25% have T2D,15,16 10% have coronary heart disease,16 5% have experienced stroke,16 and 8% have experienced myocardial infarction.16 Although the prevalence of COPD among Veterans is unknown, it is thought to be increasing, particularly among Operation Enduring Freedom/Operation Iraqi Freedom Veterans.17
Currently, only 8% of Veterans have a dental issue that is service-connected, or meet other criteria required to receive dental care through the Department of Veterans Affairs (VA).18 To help address this issue, the VA initiated the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits program. This demonstration program is designed to increase Veteran access to dental health care by connecting them with community-based, pro bono, or discounted dental service providers.18 The goal of the program is to improve Veterans’ health while also decreasing health care-related costs from emergency dental-related care by enabling VA administrative staff to coordinate community-based care for Veterans that are otherwise ineligible to receive dental care at the VA. In 2020, the VA Partnered Evidence-Based Policy Resource Center (PEPReC) was tasked with evaluating the impact of the program on Veterans’ health and health care utilization, and requested a rapid review of the impact of detection and treatment of dental problems on chronic disease outcomes to help inform this evaluation.
Detection and early treatment of dental problems, including periodontal disease, is commonly carried out through the provision of preventive dental services such as routine exams, cleanings, and education on oral hygiene. Regular access to these services may reduce painful and costly dental complications, and conceivably routine dental care and detection of dental problems may positively impact chronic disease outcomes (Figure 1). Consequently, the VA’s demonstration program could impact Veterans’ health beyond reducing dental-related emergency care if it leads to improved oral health and successful treatment of periodontal disease, and if that treatment results in improvement in chronic disease outcomes. The purpose of the current ESP review is to evaluate whether detection and treatment of dental problems has an impact on chronic disease-related outcomes for those with CVD, cerebrovascular disease, T2D, or COPD. Specifically, we focused on the detection and treatment of periodontal disease, as the link between periodontal disease and chronic disease is well evidenced and represents the area where the VA pilot program could have meaningful clinical impact.
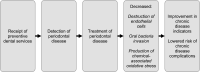
Figure 1
Conceptual Pathway between Receipt of Preventive Dental Services and Improvement in Chronic Disease Outcomes.
SCOPE
This rapid review summarizes the benefits and harms of detection and treatment of dental problems on non-dental health, quality of life, health care utilization, and costs among adults with CVD, cerebrovascular disease, T2D, and/or COPD. An analytic framework depicting these key questions and PICOTS is presented in Figure 2.
KEY QUESTIONS
- Key Question 1.
Among adults with CVD, cerebrovascular disease, T2D, and/or COPD, does detection and treatment of dental problems improve patient-reported symptoms and other complications of chronic disease?
- Key Question 2.
Among adults with CVD, cerebrovascular disease, T2D, and/or COPD, does detection and treatment of dental problems improve indicators of chronic disease management (eg, HbA1c, blood pressure, cholesterol) and patient quality of life?
- Key Question 3.
Among adults with CVD, cerebrovascular disease, T2D, and/or COPD, does detection and treatment of dental problems decrease health care utilization and costs?
- Key Question 4.
Among adults with CVD, cerebrovascular disease, T2D, and/or COPD, what are the possible harms of detection and treatment of dental problems?
ELIGIBILITY CRITERIA
The ESP included studies that met the following criteria:
- Population: Adults (excluding pregnant women) with CVD, cerebrovascular disease, T2D, and/or COPD
- Intervention: Detection and treatment of dental problems (ie, use of preventive dental services such as regular oral exams, detection of dental problems, and treatment of dental problems detected during exams)
- Comparator: No detection or treatment of dental problems
- Outcomes:
- Clinical outcomes (eg, patient-reported symptoms, complications of chronic diseases)
- Chronic disease indicators (eg, HbA1c, blood pressure, cholesterol)
- Quality of life (eg, oral health-related quality of life, overall quality of life)
- Healthcare utilization (eg, emergency department visits for non-traumatic dental conditions or non-dental conditions, health care visits associated with chronic disease management, direct costs)
- Harms (Any)
- Timing: Any
- Setting: Any
- Study design: Using a best-evidence approach, we prioritized evidence from systematic reviews (SRs) and multisite comparative studies that adequately controlled for potential patient-, provider-, and system-level confounding factors. Inferior study designs (eg, single-site, inadequate control for confounding, noncomparative) were only included when they filled gaps in higher-level evidence

Figure 2
Analytic Framework of Key Questions and PICOs.
METHODS
SEARCHES AND STUDY SELECTION
We conducted a 2-stage search to identify studies addressing our key questions. In the first stage, a research librarian searched Ovid MEDLINE, Cochrane Database of Systematic Reviews (CDSR), and other SR databases for SRs published from database inception to October 2020 using terms for dental care and chronic diseases (ie, CVD, cerebrovascular disease, diabetes, or COPD). In the second stage, the research librarian searched Ovid MEDLINE and CENTRAL from database inception to December 2020 for primary studies of PICOTS not addressed by SRs, and from January 2019 to December 2020 for primary studies published since the end date of the most recent and relevant SRs’ searches.
The first stage of the search identified several SRs on the relation of periodontal treatment to CVD and T2D clinical outcomes. We did not identify any SRs addressing cerebrovascular disease or COPD, non-clinical outcomes (eg, quality of life, health care utilization, or costs) for any chronic disease, or interventions other than periodontal treatment. As a result, 2 gap searches were conducted to identify primary studies examining the relation of periodontal treatment to all outcomes among those with cerebrovascular disease or COPD, and the relation of periodontal treatment to health care utilization, costs, quality of life, complications, and patient-reported symptoms among those with diabetes or CVD. A third search was conducted from January 2019 to Dec 2020 to identify primary studies, published since the end date of the most recent and relevant SRs’ searches, related to periodontal treatment and clinical outcomes for those with diabetes or CVD. Complete search strategies for the SR and primary study searches are described in the Supplementary Materials.
We limited the interventions included in gap searches to periodontal treatment for 2 reasons. First, all SRs identified in the first stage focused on periodontal treatment (and not other interventions, such as dental cleaning or periodic exams). Second, although periodontal disease may affect chronic diseases through several mechanisms, detection and treatment of periodontal disease is the most likely intervention through which dental services may improve chronic disease management and patient quality of life (see Background section and Figure 1). Based on these observations, we limited the review scope to evidence of the direct association between periodontal treatment and chronic disease outcomes.
Additional citations were identified from hand searching reference lists and consultation with content experts. We limited the search to published and indexed articles involving human subjects available in the English language. Study selection was based on the eligibility criteria described above (see Supplementary Materials for full inclusion/exclusion criteria). SRs had to meet 4 criteria established by the AHRQ Evidence-based Practice Program19 to merit inclusion: 1) have an explicit and adequate search, 2) apply predefined eligibility criteria to select studies, 3) conduct risk of bias assessment for included studies, and 4) present a synthesis of results. All titles, abstracts, and full-text articles were reviewed by 1 investigator and checked by another. All disagreements were resolved by consensus.
QUALITY ASSESSMENT AND DATA EXTRACTION
Given the large number of SRs published on this topic, we used guidance from the AHRQ Evidence-based Practice Center Program19 to prioritize the most recent and relevant SRs to discuss in our review. We then used predefined criteria from AMSTAR-2 to assess the quality of prioritized SRs20 and gave final assessments of high (no or 1 non-critical weakness), moderate (more than 1 non-critical weakness), low (1 critical flaw with or without non-critical weaknesses), or critically low quality (more than 1 critical flaw with or without non-critical weaknesses). We included all primary studies that addressed gaps in evidence from our prioritized SRs. We used Cochrane’s Risk of Bias Tool 2.0 to rate the quality of randomized controlled trials,21 and Cochrane’s ROBINS-I tool to rate the quality of observational studies with control groups, and gave final assessments of high, fair, or low quality.22 For observational studies without control groups or modeling studies, we informally assessed study limitations. We abstracted data from all prioritized SRs and all included primary studies as well as results for each included outcome. All data abstraction and quality ratings were first completed by 1 reviewer then checked by another. All disagreements were resolved by consensus.
STRENGTH OF EVIDENCE ASSESSMENT
We graded the strength of the evidence for categories of outcomes based on the AHRQ Methods Guide for Comparative Effectiveness Reviews.23 This approach incorporates 4 key domains: risk of bias (includes study design and aggregate quality), consistency, directness, and precision of the evidence. It also considers other optional domains that may be relevant for some scenarios, such as a dose-response association, plausible confounding that would decrease the observed effect, strength of association (magnitude of effect), and publication bias. Strength of evidence is graded for each key outcome measure, with ratings ranging from high to insufficient, reflecting our confidence that the evidence represents true intervention effects.
We used the following algorithm to make our assessments. Findings supported by a high-quality SR of multiple RCTs (or these RCTs alone) with consistent findings and either no or only minor methodological limitations of included RCTs were rated as high strength of evidence. Findings supported by a moderate- or high-quality SR of multiple RCTs (or these RCTs alone) with consistent findings but some methodological limitations of included RCTs were rated as moderate strength of evidence. Findings supported by a moderate- or high-quality SR of multiple RCTs (or these RCTs alone) with inconsistent findings or significant methodological limitations of included RCTs, or findings supported by a single good- or fair-quality RCT or controlled observational study, were rated as low strength evidence. Findings supported by a single poor-quality controlled study, uncontrolled studies, or for which no studies were available were rated as insufficient strength of evidence.
All strength of evidence ratings were first completed by 1 reviewer then checked by another. All disagreements were resolved by consensus.
SYNTHESIS OF DATA
Available evidence on those with type 2 diabetes and CVD was synthesized narratively due to the inclusion of both SRs and primary research studies. We also synthesized evidence on those with COPD and cerebrovascular disease narratively. Although we identified no SRs on these conditions, primary studies were highly variable in terms of study designs, outcome measurements, and timing of outcome measurements, which precluded us from synthesizing results quantitatively. Findings of included SRs and primary studies (where applicable) were summarized separately for each chronic disease.
The complete description of our methods can be found on the PROSPERO international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO/; registration number CRD42020215625).
RESULTS
The literature flow diagram (Figure 3) summarizes the results of the search and study selection processes. Among 520 potentially relevant SR citations and 1,248 potentially relevant primary study citations, we included 46 studies (25 SRs24–48 and 21 primary studies49–69). Of the 25 included SRs, we prioritized 8 that were the most recent and relevant: 2 review of reviews25,30 (1 with meta-analysis), 2 Cochrane SRs38,43 (1 with meta-analysis), and 4 other SRs26,32,37,48 (2 with meta-analysis). The remaining 17 SRs were not prioritized because they were already included in a prioritized review of reviews, their PICOs of interest were otherwise covered by more recent or relevant reviews, or they did not report data on clinically relevant outcomes (see Supplementary Materials for a list of these 17 SRs and specific reasons why each was not prioritized). The 21 primary studies included from our gap search and update search consisted of 8 RCTs,49,50,60,65–69 1 non-randomized controlled trial,56 8 retrospective cohort studies,52,53,55,57,59,61,62,64 1 case-control study,58 2 modeling studies,51,63 and 1 self-controlled case-series.54
We found the largest volume of evidence for people with T2D (6 reviews plus 17 primary studies), followed by CVD (2 reviews plus 5 primary studies), cerebrovascular disease (3 primary studies), and COPD (4 primary studies). Most SRs and primary studies examined non-surgical periodontal treatment (eg, scaling and root planing with or without adjunctive treatments such as antibiotics or oral hygiene instructions) although a few SRs included studies that looked at surgical treatment. Comparators generally consisted of no periodontal treatment, delayed treatment, or less-intensive forms of care (eg, oral health instructions only or community care). Across studies, outcomes relevant to all 4 of our key questions were assessed, including 1) patient-reported symptoms and complications of chronic disease (eg, oral health-related quality of life [OHRQoL], frequency of COPD exacerbations, risk of stroke or myocardial infarction); 2) markers of inflammation and chronic disease severity (eg, HbA1c, fasting blood glucose, cholesterol, triglycerides, IL-6, and CRP); 3) health care utilization and costs (eg, inpatient admissions, medical costs); and 4) harms of periodontal treatment.
In terms of quality assessment, 2 SRs (both Cochrane reviews) were high quality, 4 were moderate quality, 1 was low quality, and 1 was critically low quality. Among moderate-quality reviews, common methodological limitations included lack of grey literature searches, absence of a publicly available review protocol, and failure to discuss individual studies’ risks of bias when interpreting results. Low- and critically low-quality reviews also provided insufficient information on data extraction or quality assessment processes. The quality of primary studies ranged from fair to low-quality. Among RCTs, patients and providers were commonly aware of group assignment (ie, unblinded), half of RCTs did not have a publicly available protocol, and there was a lack of information on cointerventions that may have affected results (ie, tooth brushing). Among cohort studies and other types of controlled, observational studies, limitations included poorly defined treatment or control groups (ie, it was often unclear if people who did not receive periodontal treatment had periodontal disease or were periodontally healthy), inadequate adjustment for differences between treatment and control groups at baseline, and a high likelihood that measured or unmeasured confounders could have influenced results.
A list of excluded studies and study-level data abstraction and quality assessment for included studies appear in the Supplementary Materials.
LITERATURE FLOW
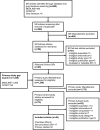
Figure 3
Literature Flowchart.
TYPE 2 DIABETES
Overview
Six SRs25,26,30,32,37,43 provide the best available evidence on the link between periodontal treatment and chronic disease indicators for those with T2D, as well as some limited information on other clinically important outcomes (Table 2). Overall, periodontal treatment was associated with small improvements in HbA1c,25,26,30,43 fasting blood glucose,25 triglycerides,32 total cholesterol,32 and CRP26 for those with T2D for up to 4 months compared to no or control treatment; however, improvements in most outcomes did not persist at 6 months. Periodontal treatment was not associated with improvements in LDL or HDL cholesterol at 3 or 6 months32 compared to no or control treatment in patients with T2D, and had mixed results (ranging from no improvement to small improvement favoring periodontal treatment) on other markers of systemic inflammation compared to no or control treatment.30,43 One SR looked at diabetes-related complications, quality of life, and cost outcomes, but found no evidence.43 Data on harms were sparse but suggested that periodontal treatment may be associated with minor adverse events (eg, diarrhea, headaches, and nausea/vomiting).
Seventeen primary studies50–53,55,57–59,61–69 (6 RCTs, 8 retrospective cohort, 1 case-control, and 2 modeling studies) address gaps in SRs or update evidence from SRs (Table 2). Overall, 5 RCTs provided updated data on chronic disease indicators and were generally in agreement with findings from SRs. The remaining 12 studies (1 RCT, 8 retrospective cohort, 1 case-control, and 2 modeling studies) examined outcomes not covered by SRs and generally had unclear results, with some studies reporting periodontal treatment is associated with improvements in oral health-related quality of life (OHRQoL),58 decreased medical costs,51,57,59,52 and decreased risk of stroke and myocardial infarction, while others reported no improvements in OHRQoL,50 increased or no change in medical costs,51,59 and no significant difference in stroke or myocardial infarction risk compared to no treatment. A single retrospective cohort study53 reported periodontal treatment is associated with reduced risk of heart failure compared to no treatment. Two studies had conflicting results on health care utilization, with one57 finding periodontal treatment was associated with lower rates of inpatient admissions for those who received periodontal treatment compared to no treatment, and another61 finding no significant difference in health care utilization between groups. Finally, a simulation study63 projected that expanding periodontal treatment coverage would lead to lower risk of diabetes-related complications.
Patient-reported Symptoms and Complications
A high-quality Cochrane review43 found no studies that reported on diabetes-related complications or quality of life. No other SRs examined this outcome.
Table 2
Overview of Best Available Evidence on Periodontal Treatment vs No Treatment Among Adults With T2 Diabetes.
A fair-quality RCT50 found that among those with T2D, there were no significant differences in OHRQoL (measured by the General Oral Health Assessment) between periodontal treatment and no treatment at 3 months, and another69 found no difference in diabetes treatment-related QoL between groups at 3 months. By contrast, a fair-quality case-control study58 found that patients with poorly-controlled T2D (HbA1c ≥ 7%) who received periodontal treatment had better OHRQoL compared to those who did not receive treatment (6.05 vs 9.02 on the Oral Health Impact Profile-14; p<.05). However, among those with well-controlled diabetes (HbA1c < 7%), there was no significant difference between groups. A major limitation of this study is that it was unclear what type of periodontal treatment patients received or how long after receiving treatment outcomes were measured. There was also no significant difference between groups on measures of functionality (SF-36) for any patients in the RCT.50
In terms of complications, a fair-quality retrospective cohort study53 found that advanced periodontal treatment was associated with a reduction in the rates of myocardial infarction (hazard ratio [HR] = 0.92, 95% CI [0.85, 0.99]) and heart failure (HR = 0.60, 95% CI [0.45, 0.80]) but not stroke (HR = 0.95, 95% CI [0.85, 1.06]) compared to non-advanced periodontal treatment over a maximum follow-up of 3 years. By contrast, a poor-quality retrospective cohort study55 found that among those with diabetes mellitus and periodontal disease, the stroke incidence rate was significantly lower in the intensive treatment (.88%/yr) than the no treatment group (1.08%/yr) over a maximum follow-up of 10 years (p<.001). A study63 that used a computer-based simulation model to project reductions of complications when periodontal treatment coverage is expanded among those with T2 diabetes (from 28% to 88% coverage) found expanded periodontal treatment coverage was associated with 7.3% reduced nephropathy incidence (95% CI [−20.3, −0.3]), 17.7% reduced neuropathy incidence (95% CI [−32.7, −4.7%]), and 18.4% reduced retinopathy incidence (95% CI [−34.5, −3.5%]) among those with T2 diabetes. Stroke and myocardial infarction incidence were also projected to be reduced by 5% (95% CI [−20.8, 3.9%]) but the difference was not statistically significant.
Overall, findings on the relation between periodontal treatment and patient-reported symptoms and complications for those with T2D are supported by low strength of evidence, as results were inconsistent across studies and studies were primarily observational with important methodological limitations. Additionally, results from the simulation study should be interpreted with caution because they were projected using a mathematical model that required the authors to make a number of assumptions about the link between periodontal treatment and diabetes-related complications that have not been directly evaluated.
Chronic Disease Indicators
A moderate-quality review of reviews25 found periodontal treatment was associated with a small but significant improvement in mean HbA1c levels at 3-6 months when compared to no treatment (weighted mean difference [WMD] = −0.32%, 95% CI [−0.5, −0.15]). A high-quality Cochrane review43 of RCTs found similar results for HbA1c at 3-4 months (MD = −0.29%, 95% CI [−0.48, −0.10]) but noted mean HbA1c levels were no longer significantly different between groups at 6 months. The review of reviews25 also reported periodontal treatment was associated with improvements in fasting blood glucose at 3-6 months when compared to no treatment (WMD = −11.59 ml/dl, 95% CI [−15.2, −8.0]), but did not evaluate whether outcomes differed at 3 or 6 months. Five RCTs65–69 published after these SRs found similar results for HbA1c.
Periodontal treatment was also associated with shorter-term improvements in total cholesterol, triglycerides, and CRP levels when compared to no or control treatment. A moderate-quality review32 reported periodontal treatment was associated with improvements in total cholesterol (mean difference [MD] = −0.47 mmol/L, 95% CI [−0.75, −0.18]) and triglycerides (MD = −0.2 mmol/L, 95% CI [−0.24, −0.16]) levels compared to no or control treatment, while the control arm was associated with improvements in HDL cholesterol (MD = 0.06 mmol/L, 95% CI [0.03, 0.08]) levels compared to periodontal treatment, all at 3 months. However, the review found no significant differences between groups at 6 months. Another moderate-quality review26 found a significant reduction in mean CRP level from baseline among those receiving periodontal treatment compared to no treatment (difference in mean change scores = 1.89 mg/L, 95% CI [1.70, 2.08]) at 3-6 months. This SR did not evaluate whether outcomes differed at 3 or 6 months. However, an RCT68 published after this SR found no significant improvements in CRP between the treatment and no treatment groups at 6 months. Individual, fair-quality RCTs additionally found periodontal treatment was associated with significantly better post-prandial glucose (PPG) at 3 months,65 improvements in oxidative stress at 3 months,69 and improvement in some measures of cardiac health (E/e’ ratio) but not others (left ventricle mass index and NT-proBNP) at 6 months68 compared to no treatment.
Finally, a moderate-quality review32 found no significant differences in mean LDL cholesterol levels between periodontal treatment and no treatment or control treatment groups at 3 or 6 months. A low-quality review37 reported that 7 studies found periodontal treatment was associated with improvements in mean IL-6 levels, while 9 studies did not find any impact (follow-up time not reported). An RCT68 published since the review found no significant difference in IL-6 between periodontal treatment and no treatment at 6 months. Finally, a critically low-quality review of reviews30 found mixed results on the relation of periodontal therapy to measures of systematic inflammation (follow-up time not reported).
Overall, findings on the relation between periodontal treatment and chronic disease indicators for those with T2D are supported by moderate strength of evidence. Although we identified several moderate- and high-quality SRs of RCTs examining these outcomes, SR authors noted that the RCTs that composed the reviews had some methodological limitations including a lack of masking of participants and study personnel as well as incomplete outcome data.
Health Care Utilization and Costs
A high-quality Cochrane review43 found no studies that reported data on costs. No other SRs examined this outcome, and no SRs examined health care utilization. Findings on costs from primary studies of primarily insurance claims data have mixed results, with studies indicating that periodontal treatment was associated with higher, lower, and no significant differences in costs compared to no periodontal treatment.
First, 5 studies52,57,61,62,64 found periodontal treatment was associated with lower costs compared to no treatment. A fair-quality retrospective cohort study52 conducted in the Netherlands found a small reduction in diabetes-related health care costs per quarter per year (−€12.03, 95% CI [−15.77, −8.29]) following periodontal treatment compared with no periodontal treatment. Several additional, poor-quality retrospective studies61,62,64 based on claims data found similar results. One of these was a US-based retrospective cohort study61 that found that, among those with newly diagnosed T2D, periodontal treatment was associated with lower healthcare costs compared to no treatment ($1,799 lower over 2 years, p=.01), although benefits were limited to those who did not initiate diabetes medications after diagnosis. Another was a US-based retrospective cohort study62 that found that people with T2D who received periodontal care had $1,750 lower annual medical costs than those who did not (statistical significance not evaluated). A third was a retrospective cohort study57 based in the US which found that those with T2D who received periodontal treatment had significantly lower mean annual medical costs than those who did not receive treatment ($4,216 vs $7,056; p<.04). A final study63 that used a computer-based simulation model to project costs of expanding periodontal treatment coverage among those with T2D (from 28% to 88% coverage) found that expanded coverage was associated with savings of $5,904 (95% CI [−6,039, −5,769]) per capita.
By contrast, 2 studies51,59 found periodontal treatment was associated with higher costs compared to no treatment. A fair-quality, retrospective cohort study based on claims data59 conducted in the US found that after adjusting for disease burden, those with diabetes mellitus who received periodontitis or gingivitis treatment had higher per-member per-year medical costs than those who received other dental services or no dental services (adjusted costs not reported). This finding was echoed by a second modeling study51 conducted in the UK that found medical costs for people with T2D who received periodontal treatment was higher than those that did not receive treatment (incremental costs ranged from £504 to £1,056). Authors pointed out that the intervention was most cost-effective for those who are older and who have higher HbA1c. A final, poor-quality retrospective cohort study64 found no significant differences in medical costs for those with newly diagnosed T2D who undergo periodontal treatment versus those who do not.
Studies also had unclear results in terms of health care utilization. One poor-quality retrospective cohort study57 found that receipt of periodontal treatment was associated with lower rates of inpatient admissions compared to controls (40.4 vs 66.6 inpatient admissions/1,000 subjects/year; p<.05), while another61 found no significant difference between groups in number of outpatient physician visits, probability of hospitalization, or occurrence of an emergency room visits.
These studies had major limitations which reduce our certainty in the findings. First, for most studies based on claims data, it was unclear whether people in the control group who did not receive periodontal treatment had periodontal disease or were periodontally healthy. It is therefore possible that confounders associated with periodontal disease – rather than periodontal treatment itself – influenced the results. One study57 attempted to control for periodontal disease status by including people who had received 1, 2, or 3 sessions of periodontal treatment in the control group (compared to 4 sessions in the intervention group). Consequently, study groups may actually represent levels of adherence to treatment rather than a comparison between receipt versus no receipt of treatment. A second limitation of the studies of claims data is that most only evaluated data from participants who were continually enrolled in both medical and dental insurance and who were alive for the duration of the study. Authors of 1 study64 commented that this may have excluded younger patients (who may have changed their health insurance) and older patients (who may have died during the study). Authors of another study61 explicitly stated that selection bias could have influenced their results, given that limiting participants to those continually enrolled in insurance reduced the sample size to around 15% of the total sample.
Overall, findings on the relation between periodontal treatment and health care utilization and costs for those with T2D are supported by low strength of evidence, because results were inconsistent across studies and studies were primarily observational with important methodological limitations.
Harms
A high-quality Cochrane review43 found that most of its included studies (20/35) did not report on adverse events, and several others (11/35) reported that either no adverse events or no major adverse events occurred. Of the remaining 4 studies, 1 study reported no adverse events occurred from use of doxycycline and 3 studies reported minor adverse events due to treatment in both groups (including diarrhea, headaches, nausea/vomiting) or due to doxycycline or chlorhexidine (including diarrhea, pain, nausea, taste change, and tooth stain). None of the included studies in another moderate-quality review26 reported on the occurrence of adverse effects or complications of periodontal treatment. Two fair-quality RCTs66,69 published since these reviews also reported there were no harms of periodontal treatment. Overall, findings on the relation between periodontal treatment and harms for those with T2D are supported by moderate strength of evidence. Importantly, although evidence is available on harms from a high-quality SR and 2 additional fair-quality RCTs, only half the studies in the included SR reported on this outcome.
CARDIOVASCULAR DISEASE
Overview
Two SRs provide the best available evidence on the link between periodontal treatment and chronic disease indicators among those CVD (Table 3). Overall, for those which chronic heart disease, periodontal treatment was associated with improvements in TNF-α, IL-6 and CRP at 3 months compared to no treatment. For those with hyperlipidemia, periodontal treatment was also associated with improvements in LDL and CRP at 3 months compared to no treatment. Although additional clinically relevant outcomes (ie, all-cause and CVD-related mortality, cardiovascular events, and adverse events) were examined by 1 review, authors did not find sufficient data to draw conclusions.
Four primary studies (4 retrospective cohort and 1 self-controlled case series) addressed gaps in SR evidence by examining the association between periodontal treatment and cardiovascular events, health care utilization, and costs (Table 3). Overall, data on complications of CVD are unclear. One retrospective cohort study55 indicated those with hypertension and atherosclerosis who received periodontal treatment had a lower risk of stroke than those who did not receive treatment, while a self-controlled case series54 indicated the risk of myocardial infarction increased in the 4 weeks after intensive dental treatment compared to baseline then decreased over the next 20 weeks. Results on costs are also unclear, with 1 retrospective cohort study59 indicating those with coronary artery disease (CAD) had higher per-member per-month medical costs than those who did not receive treatment and 2 others57,62 indicating those with CAD had lower annual medical costs. One of these studies62 reported those with CHD similarly had lower annual medical costs than those who did not receive treatment, and the other57 reported lower inpatient admissions among those with CAD who received treatment. No SRs or primary studies reported on harms.
Patient-reported Symptoms and Complications
A high-quality Cochrane review38 looked for data on all-cause and CVD-related mortality and cardiovascular events but found only a single relevant study with high attrition. A poor-quality, retrospective cohort study55 identified in the gap search found that among those with periodontal disease and hypertension, the stroke incident rate was lower in the intensive treatment group (.83%/yr) than in the no treatment group (1.09%/yr) over a maximum follow-up of 10 years (p<.001). This study found similar results for those with atherosclerosis (incident rate of intensive treatment = 1.00%/yr vs IR of no treatment = 1.18%/yr; p<.001). A self-controlled case-series54 reported the rate of myocardial infarction was higher in the first 4 weeks after an invasive dental treatment compared with baseline (incident ratio [IR] = 1.56, 95% CI [0.98, 2.47]), but noted that this rate decreased over the subsequent 20 weeks. Overall, findings on the relation between periodontal treatment and patient-reported symptoms and complications for those with CVD are supported by insufficient strength of evidence, as studies reported conflicting results and either were poor quality or did not have a separate control group.
Chronic Disease Indicators
A moderate-quality review48 included 3 studies of periodontal treatment compared to no treatment for those with CVD. One study found those with chronic heart disease who received periodontal treatment experienced improvements in TNF-α, IL-6, and CRP levels at 3 months compared to no treatment. The second found that those with refractory hypertension who received periodontal treatment experienced improvements in IL-6 and CRP levels at 6 months, although it is unclear whether control groups also improved. The final study found that those with hyperlipidemia who received periodontal treatment experienced improvements in LDL and CRP levels compared to no treatment at 3 months. By contrast, a high-quality Cochrane review38 looked for data on blood test results for those with CVD undergoing periodontal treatment but found no usable data due to substantial attrition in the single relevant study. Overall, findings on the relation between periodontal treatment and chronic disease indicators for those with CVD are supported by low strength of evidence. Although findings are supported by a high- and a moderate-quality SR, the SRs came to different conclusions based on the small number of available studies that had important methodological limitations including high attrition.
Table 3
Overview of Best Available Evidence on Periodontal Treatment vs No Treatment Among Adults With Cardiovascular Disease.
Health Care Utilization and Costs
A poor-quality retrospective cohort study57 reported that receipt of periodontal treatment was associated with significantly lower rates of inpatient admissions compared to no treatment among people with coronary artery disease (CAD) (46.6 vs 65.2 inpatient admissions/1,000 subjects/year; p<.01). As noted above, a major limitation of this study was that the control group was active (control participants received 1, 2, or 3 sessions of periodontal treatment compared to 4 sessions in the intervention group), which could have attenuated observed treatment effects.
In terms of costs, a fair-quality retrospective cohort study59 reported that after adjusting for disease burden, people with CAD who received periodontal treatment incurred higher per-member per-month medical costs than those who received no dental services (adjusted costs not reported). By contrast, a poor-quality retrospective cohort study57 found that those with CAD who received periodontal treatment had significantly lower annual medical costs than those who did not receive treatment ($9,133 vs $10,222; p<.04). A second poor-quality retrospective cohort study62 conducted in the US found that people with CAD who received periodontal treatment had lower annual medical costs compared to those who did not receive periodontal treatment (average $15,549-$16,271 in periodontal treatment group vs $20,502-$21,202 in no treatment group depending on compliance with medical care). Authors noted similar results for those with congestive heart disease (average $35,669-$36,172 in periodontal treatment group vs $47,332-$49,064 in no treatment group depending on compliance with medical care). Differences were not evaluated for statistical significance in this study, which was published as a report by United Healthcare.
Overall, findings on the relation between periodontal treatment and healthcare utilization and costs for those with CVD are supported by low strength of evidence, as studies reported conflicting results and were all observational with important methodological limitations.
Harms
A high-quality Cochrane review38 looked for data on harms but found no usable data due to high attrition in the single relevant study. No primary studies identified in the gap search reported on harms.
CEREBROVASCULAR DISEASE
Overview
We did not identify any SRs examining the link between periodontal treatment and any outcomes of interest for those with cerebrovascular disease. Three primary studies54,57,59 (2 retrospective cohort and 1 self-controlled case series) conducted in those with cerebrovascular disease reported similar findings as those with CVD (Table 4). One retrospective cohort study59 found that after adjusting for disease burden, people with cerebrovascular disease who underwent periodontal treatment had higher medical costs than those who did not receive dental services, while another57 found they have lower annual medical costs and lower rates of inpatient admissions. A third self-controlled case series54 found that the risk of ischemic stroke was higher in the 4 weeks after intensive dental therapy than at baseline.
Patient-reported Symptoms and Complications
A self-controlled case series54 reported that the risk of stroke was slightly elevated in the 4 weeks after an invasive dental treatment (IR = 1.39, 95% CI [0.89, 2.15]) with an unclear pattern of resolution in the following weeks. Overall, this finding is supported by insufficient strength of evidence as the single observational study did not have a separate control group.
Chronic Disease Indicators
No studies reported on chronic disease indicators.
Health Care Utilization and Costs
One fair-quality retrospective cohort study59 found that after adjusting for disease burden, people with cerebrovascular disease who underwent periodontal treatment had higher medical costs than those who did not receive dental services (adjusted costs not reported). By contrast, a poor-quality retrospective cohort study57 indicated people with cerebrovascular disease who received periodontal treatment had lower mean annual medical costs compared to those who did not receive dental services ($8,214 vs $13,895; p<.04). This study also reported lower rates of inpatient admissions in the periodontal treatment group compared to the no treatment group (350.0 vs 444.4 inpatient admissions/1,000 subjects/year; p<.002). Overall, findings are supported by low strength of evidence, as studies reported conflicting results and were observational with important methodological limitations, including 1 study whose comparison group was composed of those who received 1, 2, or 3 sessions of periodontal therapy and another whose comparison group was composed of people who may have been periodontally healthy.
Harms
No primary studies reported on harms.
Table 4
Overview of Best Available Evidence on Periodontal Treatment vs No Treatment Among Adults With Cerebrovascular Disease.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
Overview
We did not identify any SRs examining the link between periodontal treatment and any outcomes of interest for those with COPD. Four primary studies49,56,60 (2 RCTs, 1 non-randomized, controlled trial, and 1 retrospective cohort study) examined outcomes of periodontal treatment versus no treatment among people with COPD (Table 5). One RCT49 and 1 non-randomized controlled trial56 found that periodontal treatment was associated with reductions in the frequency of COPD exacerbations at 1 and 2 years, while another, shorter RCT60 indicated that periodontal treatment had no significant effect on COPD-related quality of life, self-assessment of health, or illness severity at 1 month. This RCT60 also found a reduction in doctor visits in both treatment and control groups (significance not assessed) and no adverse events in either group. The retrospective cohort study62 found lower annual medical costs among those who received periodontal treatment compared to those who did not.
Patient-reported Symptoms and Complications
A fair-quality RCT49 (N=60) and another fair-quality, non-randomized controlled trial56 (N=40) found that periodontal treatment was associated with significant reductions in the mean frequency of COPD exacerbations at 1 year (1.95 vs 3.25 exacerbations/patient-year) and 2 years (30% of patients experiencing frequent/70% experiencing infrequent exacerbations vs 66.7% frequent/33.3% infrequent) compared to no treatment. A shorter and smaller fair-quality RCT60 (N=30) did not find significant differences between treatment groups in COPD-related quality of life (measured by SGRQ-A), self-assessment of health, or illness severity at 1 month. These findings are supported by low strength of evidence, as evidence on short- and long-term findings conflict, and RCTs were small with important methodological limitations including 1 study that did not describe how patients were allocated to treatment versus control groups.
Chronic Disease Indicators
A fair-quality RCT49 (N=60) with 2 periodontal treatment groups (scaling and root planing or supragingival scaling treatment) found both forms of treatment had a significant, positive effect on measures of lung function (including forced expiratory volume in the first second [FEV1] and forced vital capacity [FEV1/FVC]) compared with no treatment at 2 years. These findings are supported by low strength of evidence, as evidence comes from a single, small RCT that did not mask participants and had no publicly available protocol.
Health Care Utilization, Costs, and Harms
A fair-quality RCT60 (N=30) found a reduction in doctor visits in both treatment and control groups and no adverse events in either group at 1 month. A poor-quality retrospective cohort study62 found that those with COPD who received periodontal treatment had lower annual medical costs than those who did not receive treatment ($12,938-$38,450 vs $15,817-$52,484 depending on medical compliance). These findings are supported by low strength of evidence, as evidence comes from a single, small RCT that did not mask participants and had no publicly available protocol, as well as a retrospective cohort study that did not clearly report whether people in the no treatment group had periodontal disease or were periodontally healthy.
Table 5
Overview of Best Available Evidence on Periodontal Treatment vs No Treatment Among Adults With Chronic Obstructive Pulmonary Disease.
SUMMARY AND DISCUSSION
We conducted a rapid review to synthesize evidence on benefits and harms of detection and treatment of dental problems (specifically, periodontal disease) among those with T2D, CVD, cerebrovascular disease, or COPD. Although multiple reviews3–5 have found a link between periodontal disease and chronic disease, it remains unclear whether treatment for periodontal disease can meaningfully improve chronic disease outcomes and reduce healthcare utilization and associated costs.
This review found that periodontal disease treatment may improve chronic disease outcomes for those with COPD. Results from 1 fair-quality RCT49 and 1 fair-quality non-randomized, controlled trial57 suggest periodontal treatment may improve lung function and reduce the frequency of exacerbations at 1 and 2 years compared to no treatment. Another fair-quality RCT60 found no adverse events within a month of periodontal treatment. However, in this short study, periodontal treatment did not reduce the number of doctor’s visits after treatment, nor did it appear to improve quality of life, self-assessment of health, or illness severity. It is possible that these discordant findings are due to the shorter follow-up period of this study (ie, the effect of periodontal treatment on some COPD outcomes may take longer than 1 month to become apparent). A poor-quality retrospective cohort study62 adds additional evidence indicating those who receive periodontal treatment have lower annual medical costs than those who do not receive treatment; however, results may have been confounded if the control group included people who were periodontally healthy.
Results from moderate- and high-quality SRs25,26,32,43,48 indicate periodontal treatment likely improves measures of chronic disease severity and inflammation (eg, HbA1c, fasting blood glucose, total cholesterol, triglycerides, CRP) compared to no treatment among those with T2D or cardiovascular disease in the short term (3-4 months). However, improvements do not seem to persist beyond 6 months. This may be due to the fact that periodontal treatment is meant to be a continuous preventive intervention (ie, scaling and root planing followed by routine check-ups and addressing subsequent problems that arise). A single periodontal treatment session or group of sessions may therefore be insufficient to produce durable improvement in chronic disease indicators.
Evidence is unclear on whether periodontal treatment reduces the risk of diabetes and CVD-related complications. For those with T2D, evidence from a single fair-quality retrospective cohort study53 indicates that periodontal treatment may reduce the risk of heart failure compared to no treatment. However, studies53,55,63 on myocardial infarction and stroke risk among those with T2D found conflicting results, with some studies indicating no difference in risk and others indicating lower risk among who received periodontal treatment. Additionally, a simulation study63 of people with T2 diabetes projected that expanding periodontal treatment would lead to improvements in diabetes-related complications. Authors of this study made important assumptions about the connection between periodontal treatment and diabetes outcomes that have not been directly evaluated. These assumptions could have led to findings that were favorable towards periodontal treatment.
For those with hypertension or atherosclerosis, evidence from a single, poor-quality retrospective cohort study55 indicates that periodontal treatment may reduce stroke risk. However, a self-controlled case series54 indicated that individuals’ risk for stroke and myocardial infarction increased after periodontal treatment when compared to baseline. It is possible the invasive nature of periodontal disease treatment triggers inflammation or releases bacteria into the blood stream, the effect of which is a temporary increase in vascular inflammation and/or atherosclerosis that leads to higher risk of cardiovascular-related adverse events.54,70 However, a 2011 letter published by Harvard Health that discusses this study pointed out that although the relative risk of stroke and myocardial infarction was higher after periodontal treatment than at baseline, the absolute risk of these events was low.70,71 Both this letter and a 2010 news article by Consumer Reports70 also pointed out that participants may have discontinued the use of NSAIDS, blood thinners, or antiplatelet medications to reduce bleeding due to periodontal treatment, and consequently these patients’ cardiovascular risk may have been heightened. Authors of the case series conducted sensitivity analyses to assess whether medication use may have confounded results but acknowledged their data on medications may have been insufficient to rule out confounding.
Findings on costs of periodontal treatment versus no treatment for people with T2D, coronary artery disease, or cerebrovascular disease are also unclear, with some studies52,57,61–63 indicating periodontal treatment contributes to reductions in costs and other studies51,59,64 finding no significant difference or higher medical costs associated with periodontal treatment. One poor-quality retrospective cohort study57 reported periodontal treatment was associated with lower inpatient admissions for those with T2D, coronary artery disease, or cerebrovascular disease, while another61 conducted in those with T2D found no significant differences between groups in total outpatient physician visits, likelihood of a hospitalization, or occurrences of emergency room visits. A major limitation of many of these studies is that the purportedly “no treatment” control groups were poorly described and may have included people who were periodontally healthy. It is reasonable to expect people who are periodontally healthy to have lower health care costs than those who have periodontal disease, and the inclusion of healthy individuals in control groups assumed to represent individuals with periodontal disease not receiving treatment could leads to biased or inaccurate findings. An additional consideration in the applicability of these studies is that several included the costs of providing periodontal treatment. Within the VA’s current pilot program, the VA would not bear the costs of periodontal treatment, which means that the costs measured in included studies are not the same as those incurred by the VA (eg, the costs associated with facilitating access to periodontal treatment or treating dental emergencies subsequent to untreated dental conditions).
Finally, we found some evidence that the balance of benefits and costs may differ by individual patient characteristics. For example, in 1 study51 that reported periodontal treatment was associated with higher medical costs compared to no treatment, authors noted that periodontal treatment was most cost-effective in those with higher HbA1c (who have more to gain from periodontal treatment-associated improvements in HbA1c) and those who are older (who have lower lifetime costs of periodontal treatment). Another study58 found that those with poorly controlled T2D (HbA1c ≥ 7%) who underwent periodontal treatment experienced significant improvements in OHRQoL compared to controls, while those with well-controlled T2D (HbA1c<7%) who underwent periodontal treatment experienced no improvements compared to controls.
LIMITATIONS
There are limitations to our rapid review methodology and to included SRs and primary studies.
Rapid Review Limitations
The primary limitation of our rapid review methodology is that we prioritized synthesis of the best available evidence, rather than all available evidence. This approach included: 1) synthesizing the 8 most recent and relevant SRs rather than all 25 SRs that met inclusion criteria; 2) conducting primary study searches to address gaps in the SR literature and primary studies published since the end date of prioritized SRs’ searches rather than all possible primary studies; and 3) having a single reviewer assess study eligibility, study quality, and strength of evidence with second reviewer checking instead of dual, independent review. It is possible that because of these steps, we missed eligible studies or eligible data; however, we likely captured the most recent and directly relevant data available on this topic.
Limitations of Included Studies
As discussed in the Results section, only 2 of the 8 prioritized SRs were high quality. Four others were moderate quality, 1 was low quality, and 1 was critically low quality. Common methodological limitations of SRs included not searching for grey literature, no publicly available protocol, no discussion of individual studies’ risk of bias, and lack of information on quality assessment and data extraction processes. Among the 21 primary studies, none were high quality, 13 were fair quality, 5 were poor quality, and 3 were not evaluated formally because they lacked a separate control group (1 was a self-controlled case series and 2 were modeling studies). The most significant limitations of RCTs were that patients and providers were aware of group assignment (unblinded). Among other types of controlled studies, treatment or control groups were poorly defined, there were potentially important differences between groups at baseline, or there was a high risk that confounders could have influenced results.
GAPS AND FUTURE RESEARCH
We generally found unclear evidence from studies with important methodological limitations, suggesting a need for better-conducted studies on this topic. Below we provide specific recommendations for future research:
- Populations: There is limited available evidence on the relation between periodontal treatment and chronic disease outcomes for those with cerebrovascular disease. Given periodontal treatment may temporarily increase the risk of adverse events such as myocardial infarction and stroke, researchers should carefully select participants for whom the long-term benefits of periodontal treatment are likely to outweigh the short-term risks. Among those with T2D, CVD, and COPD, researchers should evaluate for which subgroups of patients does periodontal treatment improve chronic disease outcomes the most. We found evidence suggesting particular subgroups of patients with T2D (ie, those with HbA1c levels ≥ 7% or older adults) may benefit the most from periodontal treatment. Similarly, researchers should evaluate whether outcomes for those with CVD or COPD vary by patient characteristics (eg, age, sex, race/ethnicity), disease severity, and whether patients have single versus multiple chronic diseases.
- Interventions: This review focused on periodontal treatment as it was the most well-evidenced intervention with respect to impact on chronic disease outcomes. We did not evaluate other interventions that fall on the potential causal pathway shown in Figure 1 and that may indirectly affect chronic disease outcomes, such as routine dental cleanings. Along these lines, researchers of the Care Coordination for Dental Benefits program may be interested in evaluating less-direct but clinically important questions such as whether referral to dental care improves chronic disease outcomes and their associated costs. In this case, it would be important to track whether referral to dental care results in increased receipt of dental services, what kinds of services are delivered (eg, routine cleanings, tooth extraction, filling cavities, scaling and root planing, and/or surgery), and whether participants continue to receive dental services on a regular basis. Important cointerventions that should be measured include participants’ oral hygiene behaviors (eg, teeth brushing, flossing, attending routine visits as recommended), as well as other interventions that may affect chronic disease outcomes (eg, medications and lifestyle interventions).
- Comparators: If the primary intervention of interest is referral to dental care services, the primary comparator of interest is no referral or delayed referral. For both intervention and control groups, it would be important to measure and account for participants having private dental insurance and/or receiving dental services outside the VA’s referral program.
- Outcomes: Because there is limited or unclear evidence on the relation of periodontal treatment with chronic disease-related complications, health care utilization, and costs, these outcomes should be evaluated in future research. Although periodontal treatment appears to have only a short-term effect on HbA1c (and therefore is unlikely to lead to long-term reductions in complications associated with poorly controlled HbA1c such as retinopathy and neuropathy), it is important to measure this outcome to rule out the possibility that periodontal treatment may inadvertently lead to increased complications. It is also important to measure cardiovascular and cerebrovascular disease-related outcomes such as myocardial infarction and stroke. In terms of health care utilization and costs, researchers should consider rare but expensive health care events (eg, ED visits and hospitalization for chronic disease-related complications) as well as common but less expensive health care events (eg, costs of medications, number/frequency of primary and specialty care visits). Additionally, VA researchers should evaluate relevant costs, given that the VA does not bear the costs of periodontal treatment in its pilot program.
- Timing: Future studies should assess outcomes at both shorter (3 and 6 month) and longer-term (1+ years) time points for all 4 chronic disease populations. For rare outcomes such as myocardial infarction and stroke, multiple years of follow-up may be required to detect any difference between periodontal treatment and no treatment groups.
- Settings: Dental and medical care have long been provided in separate settings; however, there is increasing interest in better coordinating medical and dental care to improve patient outcomes. For example, in 2019 the US Department of Health and Human Services (HHS) awarded $85 million to nearly 300 health centers to expand their oral health service capacity to help improve patients’ overall health and well-being.72 The VA is an ideal place to test the effect of a medical-dental care coordination program, as Veterans are generally older and have more comorbidities than the general population (suggesting dental services have a greater than average potential to improve chronic disease outcomes). The VA also has an existing care coordination infrastructure in the form of the Patient Aligned Care Team (PACT) program, which could be expanded to include medical-dental care coordination.
- Study Designs: A hybrid effectiveness-implementation trial design73 is 1 option for evaluating VA’s Care Coordination for Dental Benefits program, given the intervention will be simultaneously implemented and evaluated. Researchers may consider randomizing VA sites to referral or no referral/delayed referral groups. This more limited implementation may also be important because care coordination interventions require dedicated skilled staff time to coordinate care, as well as the development and maintenance of relationships with community-based dental providers. If it is not feasible to randomize sites, a prospective cohort study that carefully matches intervention and control group participants and measures and adjusts for potential cointerventions and confounders, would be another viable option.
CONCLUSIONS
Among people with COPD, periodontal treatment may improve lung function and reduce exacerbations at 1-2 years, as well as reduce annual medical costs. Among people with diabetes or cardiovascular disease, periodontal treatment likely leads to improvements in some measures of chronic disease severity and inflammation at 3-4 months, but benefits do not seem to persist beyond 6 months. Results are unclear on the relation between periodontal treatment and chronic disease outcomes for those with cerebrovascular disease. Results are also unclear on the relation between periodontal treatment and medical costs and risk of chronic disease complications among those with diabetes, cardiovascular disease, or cerebrovascular disease.
ACKNOWLEDGMENTS
This topic was developed in response to a nomination by the Partnered Evidence-Based Policy Resource Center (PEPReC) for the purpose of informing the implementation and evaluation of the Veterans Innovation Center (VIC) Care Coordination for Dental Benefits demonstration program. The scope was further developed with input from the topic nominators (ie, Operational Partners) and the ESP Coordinating Center.
In designing the study questions and methodology at the outset of this report, the ESP consulted several technical and content experts. Broad expertise and perspectives were sought. Divergent and conflicting opinions are common and perceived as healthy scientific discourse that results in a thoughtful, relevant systematic review. Therefore, in the end, study questions, design, methodologic approaches, and/or conclusions do not necessarily represent the views of individual technical and content experts.
The authors gratefully acknowledge Mark Helfand, MD, MPH, MS for input on review scope, Payten Sonnen for help with data extraction and editorial review, and Julia Haskin, MA for her editorial review, as well as the following individuals for their contributions to the project:
Operational Partners
Operational partners are system-level stakeholders who have requested the report to inform decision-making. They recommend Technical Expert Panel (TEP) participants; assure VA relevance; help develop and approve final project scope and timeframe for completion; provide feedback on draft report; and provide consultation on strategies for dissemination of the report to field and relevant groups.
Melissa Garrido, PhD
- Associate Director, Partnered Evidence-Based Policy Resource Center (PEPReC)
- Boston VA Healthcare System
REFERENCES
- 1.
- Periodontal Disease. Centers for Disease Control and Prevention. Oral Health Web site. https://www
.cdc.gov/oralhealth /conditions /periodontal-disease .html#:~:text=A%20recent %20CDC%20report1,some %20form%20of%20periodontal %20disease. Updated July 10, 2013. Accessed January 12, 2021. - 2.
- Oral Health in America: A Report of the Surgeon General. U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health. https://www
.nidcr.nih .gov/sites/default/files /2017-10/hck1ocv .%40www.surgeon.fullrpt.pdf. Published 2000. Accessed. - 3.
- Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association? Circulation. 2012;125(20):2520–2544. [PubMed: 22514251]
- 4.
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84:(4 Suppl):S135–152. [PubMed: 23631574]
- 5.
- Azarpazhooh A, Leake JL. Systematic Review of the Association Between Respiratory Diseases and Oral Health. Journal of Periodontology. 2006;77(9):1465–1482. [PubMed: 16945022]
- 6.
- Si Y, Fan H, Song Y, Zhou X, Zhang J, Wang Z. Association between periodontitis and chronic obstructive pulmonary disease in a Chinese population. J Periodontol. 2012;83(10):1288–1296. [PubMed: 22248220]
- 7.
- Sharma N, Shamsuddin H. Association between respiratory disease in hospitalized patients and periodontal disease: a cross-sectional study. J Periodontol. 2011;82(8):1155–1160. [PubMed: 21219100]
- 8.
- Cafasso J. Periodontitis. Healthline. https://www
.healthline .com/health/periodontitis#symptoms. Published 2017. Accessed February 19, 2021. - 9.
- Leong XF, Ng CY, Badiah B, Das S. Association between hypertension and periodontitis: possible mechanisms. Thescientificworldjournal. 2014;2014:768237. [PMC free article: PMC3910336] [PubMed: 24526921]
- 10.
- Stanko P, Izakovicova Holla L. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):35–38. [PubMed: 24509898]
- 11.
- Persson GR. Diabetes and Periodontal Disease: An Update for Health Care Providers. Diabetes Spectrum. 2011;24(4):195–198.
- 12.
- High blood pressure. American Heart Association. Understand Your Risks to Prevent a Heart Attack Web site. https://www
.heart.org /en/health-topics/heart-attack /understand-your-risks-to-prevent-a-heart-attack#:~:text=High %20blood %20pressure%20increases %20the,failure%20and %20congestive%20heart%20failure. Published 2016. Updated June 30, 2016. Accessed February, 19, 2021. - 13.
- Giacco F, Brownlee M, Schmidt AM. Oxidative Stress and Diabetic Complications. Circulation Research. 2010;107(9):1058–1070. [PMC free article: PMC2996922] [PubMed: 21030723]
- 14.
- Sperr M, Kundi M, Tursic V, et al. Prevalence of comorbidities in periodontitis patients compared with the general Austrian population. J Periodontol. 2018;89(1):19–27. [PubMed: 28844189]
- 15.
- Liu Y, Sayam S, Shao X, et al. Prevalence of and Trends in Diabetes Among Veterans, United States, 2005-2014. Centers for Disease Control and Prevention. Preventing Chronic Disease Web site. https://www
.cdc.gov/pcd /issues/2017/17_0230.htm. Published 2017. Updated December 14, 2017. Accessed February 19, 2021. - 16.
- Hinojosa R. Veterans’ Likelihood of Reporting Cardiovascular Disease. The Journal of the American Board of Family Medicine. 2019;32(1):50–57. [PubMed: 30610141]
- 17.
- Pugh MJ, Jaramillo CA, Leung K-w, et al. Increasing Prevalence of Chronic Lung Disease in Veterans of the Wars in Iraq and Afghanistan. Military Medicine. 2016;181(5):476–481. [PubMed: 27136656]
- 18.
- Pilot Program for Dental Health Care Access Department of Veterans Affairs. https://www
.govinfo.gov /content/pkg/FR-2019-12-13 /pdf/2019-26901.pdf. Published 2019. Accessed February 19, 2021. - 19.
- Robinson KA, Chou R, Berkman ND, et al. Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J Clin Epidemiol. 2016;70:38–44. [PubMed: 26261004]
- 20.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 21.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [PubMed: 31462531]
- 22.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [PMC free article: PMC5062054] [PubMed: 27733354]
- 23.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville MD2013.
- 24.
- Artese HP, Foz AM, Rabelo Mde S, et al. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PLoS ONE [Electronic Resource]. 2015;10(5):e0128344. [PMC free article: PMC4444100] [PubMed: 26010492]
- 25.
- Ata-Ali F, Melo M, Cobo T, Nagasawa MA, Shibli JA, Ata-Ali J. Does Non-Surgical Periodontal Treatment Improve Glycemic Control? A Comprehensive Review of Meta-Analyses. Journal of the International Academy of Periodontology. 2020;22(4):205–222. [PubMed: 32980833]
- 26.
- Baeza M, Morales A, Cisterna C, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. Journal of Applied Oral Science. 2020;28:e20190248. [PMC free article: PMC6919200] [PubMed: 31939522]
- 27.
- Botero JE, Rodriguez C, Agudelo-Suarez AA. Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Australian Dental Journal. 2016;61(2):134–148. [PubMed: 26815303]
- 28.
- Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019;19(1):176. [PMC free article: PMC6685286] [PubMed: 31387569]
- 29.
- Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: A systematic review and meta-analysis. Journal of Diabetes Investigation. 2013;4(5):502–509. [PMC free article: PMC4025114] [PubMed: 24843701]
- 30.
- D’Aiuto F, Gable D, Syed Z, et al. Evidence summary: The relationship between oral diseases and diabetes. British Dental Journal. 2017;222(12):944–948. [PubMed: 28642531]
- 31.
- Faggion CM, Jr., Cullinan MP, Atieh M. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. Journal of Periodontal Research. 2016;51(6):716–725. [PubMed: 26913689]
- 32.
- Garde S, Akhter R, Nguyen MA, Chow CK, Eberhard J. Periodontal Therapy for Improving Lipid Profiles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019;20(15):05. [PMC free article: PMC6695858] [PubMed: 31387283]
- 33.
- Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S. Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes. Medicina Oral, Patologia Oral y Cirugia Bucal. 2017;22(2):e167–e176. [PMC free article: PMC5359698] [PubMed: 28160589]
- 34.
- Jain A, Gupta J, Bansal D, Sood S, Gupta S, Jain A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis. Journal of Indian Society of Periodontology. 2019;23(4):303–310. [PMC free article: PMC6628769] [PubMed: 31367125]
- 35.
- Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials [Electronic Resource]. 2015;16:291. [PMC free article: PMC4490675] [PubMed: 26137892]
- 36.
- Liew AK, Punnanithinont N, Lee YC, Yang J. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Australian Dental Journal. 2013;58(3):350–357. [PubMed: 23981218]
- 37.
- Lima RPE, Belem FV, Abreu LG, et al. Effect of Periodontal Therapy on Serum Levels of IL-6 in Type 2 Diabetics: A Systematic Review. International Journal of Periodontics & Restorative Dentistry. 2019;39(1):e1–e10. [PubMed: 30543722]
- 38.
- Liu W, Cao Y, Dong L, et al. Periodontal therapy for primary or secondary prevention of cardiovascular disease in people with periodontitis. Cochrane Database of Systematic Reviews. 2019;12:CD009197. [PMC free article: PMC6953391] [PubMed: 31887786]
- 39.
- Manger D, Walshaw M, Fitzgerald R, et al. Evidence summary: the relationship between oral health and pulmonary disease. British Dental Journal. 2017;222(7):527–533. [PubMed: 28387268]
- 40.
- Mauri-Obradors E, Jane-Salas E, Sabater-Recolons Mdel M, Vinas M, Lopez-Lopez J. Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review. Odontology/The Society of the Nippon Dental University. 2015;103(3):301–313. [PubMed: 25062756]
- 41.
- Perez-Losada FL, Jane-Salas E, Sabater-Recolons MM, Estrugo-Devesa A, Segura-Egea JJ, Lopez-Lopez J. Correlation between periodontal disease management and metabolic control of type 2 diabetes mellitus. A systematic literature review. Medicina Oral, Patologia Oral y Cirugia Bucal. 2016;21(4):e440–446. [PMC free article: PMC4920457] [PubMed: 26827070]
- 42.
- Schmitt A, Carra MC, Boutouyrie P, Bouchard P. Periodontitis and arterial stiffness: a systematic review and meta-analysis. Journal of Clinical Periodontology. 2015;42(11):977–987. [PubMed: 26465940]
- 43.
- Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database of Systematic Reviews. 2015(11):CD004714. [PMC free article: PMC6486035] [PubMed: 26545069]
- 44.
- Sun QY, Feng M, Zhang MZ, et al. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: a meta-analysis of randomized controlled trials. Chinese Journal of Physiology. 2014;57(6):305–314. [PubMed: 25575518]
- 45.
- Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):421–427. [PMC free article: PMC2809296] [PubMed: 20103557]
- 46.
- Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016;17(1):31. [PMC free article: PMC4967318] [PubMed: 27473177]
- 47.
- Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2014;9(9):e108412. [PMC free article: PMC4177914] [PubMed: 25255331]
- 48.
- D’Isidoro O, Perrotti V, Hui WL, Piattelli A, Iaculli F, Quaranta A. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: A literature review. American Journal of Dentistry. 2019;32(4):191–200. [PubMed: 31436940]
- 49.
- Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. Journal of Clinical Periodontology. 2014;41(6):564–572. [PubMed: 24593836]
- 50.
- Vergnes JN, Canceill T, Vinel A, et al. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. Journal of Clinical Periodontology. 2018;45(10):1150–1163. [PubMed: 30136741]
- 51.
- Solowiej-Wedderburn J, Ide M, Pennington M. Cost-effectiveness of non-surgical periodontal therapy for patients with type 2 diabetes in the UK. Journal of Clinical Periodontology. 2017;44(7):700–707. [PubMed: 28504365]
- 52.
- Smits KPJ, Listl S, Plachokova AS, Van der Galien O, Kalmus O. Effect of periodontal treatment on diabetes-related healthcare costs: a retrospective study. BMJ Open Diabetes Research & Care. 2020;8(1):10. [PMC free article: PMC7590362] [PubMed: 33099508]
- 53.
- Peng CH, Yang YS, Chan KC, Kornelius E, Chiou JY, Huang CN. Periodontal Treatment and the Risks of Cardiovascular Disease in Patients with Type 2 Diabetes: A Retrospective Cohort Study. Internal Medicine. 2017;56(9):1015–1021. [PMC free article: PMC5478560] [PubMed: 28458305]
- 54.
- Minassian C, D’Aiuto F, Hingorani AD, Smeeth L. Invasive dental treatment and risk for vascular events: a self-controlled case series. Annals of Internal Medicine. 2010;153(8):499–506. [PubMed: 20956706]
- 55.
- Lee YL, Hu HY, Huang N, Hwang DK, Chou P, Chu D. Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke. 2013;44(4):1026–1030. [PubMed: 23422085]
- 56.
- Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. Journal of Periodontology. 2013;84(7):863–870. [PubMed: 23003917]
- 57.
- Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. American Journal of Preventive Medicine. 2014;47(2):166–174. [PubMed: 24953519]
- 58.
- Hsu YJ, Lin KD, Chen JH, et al. Periodontal Treatment Experience Associated with Oral Health-Related Quality of Life in Patients with Poor Glycemic Control in Type 2 Diabetes: A Case-Control Study. International Journal of Environmental Research & Public Health [Electronic Resource]. 2019;16(20):19. [PMC free article: PMC6843950] [PubMed: 31635118]
- 59.
- Albert DA, Sadowsky D, Papapanou P, Conicella ML, Ward A. An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Services Research. 2006;6:103. [PMC free article: PMC1574303] [PubMed: 16914052]
- 60.
- Agado BE, Crawford B, DeLaRosa J, et al. Effects of periodontal instrumentation on quality of life and illness in patients with chronic obstructive pulmonary disease: a pilot study. Journal of Dental Hygiene. 2012;86(3):204–214. [PubMed: 22947843]
- 61.
- Nasseh K, Vujicic M, Glick M. The Relationship between Periodontal Interventions and Healthcare Costs and Utilization. Evidence from an Integrated Dental, Medical, and Pharmacy Commercial Claims Database. Health Economics. 2017;26(4):519–527. [PMC free article: PMC5347922] [PubMed: 26799518]
- 62.
- Healthcare U. Medical Dental Integration Study. https://www
.unitedhealthgroup .com/content /dam/UHG/PDF/2013/UHC-Medical-Dental-Integration-Study.pdf. Published 2013. Accessed February 19, 2021. - 63.
- Choi SE, Sima C, Pandya A. Impact of treating oral disease on preventing vascular diseases: a model-based cost-effectiveness analysis of periodontal treatment among patients with type 2 diabetes. Diabetes care. 2020;43(3):563–571. [PubMed: 31882408]
- 64.
- Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: Evidence from a German claims database. Diabetes Research and Clinical Practice. 2021;172:108641. [PubMed: 33359573]
- 65.
- Das AC, Das SJ, Panda S, Sharma D, Taschieri S, MD. F. Adjunctive Effect of Doxycycline with Conventional Periodontal Therapy on Glycemic Level for Chronic Periodontitis with Type 2 Diabetes Mellitus Subjects. Journal of Contemporary Dental Practice [Electronic Resource]. 2019;20(12):1417–1423. [PubMed: 32381843]
- 66.
- El-Makaky Y, Shalaby HK. The effects of non-surgical periodontal therapy on glycemic control in diabetic patients: A randomized controlled trial. Oral Diseases. 2020;26(4):822–829. [PubMed: 31834660]
- 67.
- Lee JY, Choi YY, Choi Y, Jin BH. Efficacy of non-surgical treatment accompanied by professional toothbrushing in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Journal of Periodontal & Implant Science. 2020;50(2):83–96. [PMC free article: PMC7192821] [PubMed: 32395387]
- 68.
- Wang Y, Liu HN, Zhen Z, et al. A randomized controlled trial of the effects of non-surgical periodontal therapy on cardiac function assessed by echocardiography in type 2 diabetic patients. Journal of clinical periodontology. 2020. [PubMed: 32350903]
- 69.
- Mizuno H, Ekuni D, Maruyama T, et al. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial. Plos one. 2017;12(11). [PMC free article: PMC5689834] [PubMed: 29145468]
- 70.
- Could dentists damage your heart while fixing your teeth? Consumer Reports. https://www
.consumerreports .org/cro/news /2010/10/could-dentists-damage-your-heart-while-fixing-your-teeth/index.htm. Published 2010. Updated October 25, 2010. Accessed January 13, 2021. - 71.
- Protect your heart during dental work. Harvard Health Publishing, Harvard Medical School https://www
.health.harvard .edu/heart-health /protect-your-heart-during-dental-work#:~:text =Researchers %20in%20England%20created %20a,any%20other %20time%20during%20the. Published 2011. Updated February, 2011. Accessed Jnauary 12, 2021. - 72.
- HHS Awards over $85 Million to Help Health Centers Expand Access to Oral Health Care. U.S. Health and Human Services. https://www
.hhs.gov/about /news/2019/09/18 /hhs-awards-over-85-million-help-health-centers-expand-access-oral-healthcare .html. Published 2018. Updated 2018. Accessed January 12, 2021. - 73.
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. [PMC free article: PMC3731143] [PubMed: 22310560]
Supplementary Materials
SEARCH STRATEGIES
SYSTEMATIC REVIEW SEARCH: OVID MEDLINE (10-14-20)
Search for current systematic reviews MEDLINE Date Searched: 10-14-20 | ||
|---|---|---|
| # | Search Statement | Results: |
| 1 | exp Dental Care/ or Dental Care for Chronically Ill/ or Preventive Dentistry/ or exp Oral Hygiene/ or Oral Health/ or exp Periodontal Diseases/ or exp Dental Caries/ | 173818 |
| 2 | (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or oral health or dental hygiene or oral hygiene or periodont* or parodontos* or pyorrhea alveolaris or gingivitis or gingiva* or paradont* or dental decay or dental caries or carious dentin$1 or root decay).ti,ab. | 172096 |
| 3 | 1 or 2 | 252602 |
| 4 | exp Cardiovascular Diseases/ or exp Cerebrovascular Disorders/ or exp Diabetes Mellitus/ or exp Pulmonary Disease, Chronic Obstructive/ | 2761864 |
| 5 | (((cardiovascular or heart) adj2 disease$1) or atheroscleros* or arterioscleros*).ti,ab. | 447365 |
| 6 | ((cerebrovascular adj2 (disorder$1 or disease$1 or occlusion$1 or insufficienc*)) or intracranial vascular disease$1 or intracranial vascular disorder$1 or brain vascular disorder$1 or stroke).ti,ab. | 276591 |
| 7 | (diabetes or diabetic or glycemic control).ti,ab. | 666350 |
| 8 | (COPD or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic obstructive pulmonary disease or chronic airflow obstruction).ti,ab. | 70849 |
| 9 | 4 or 5 or 6 or 7 or 8 | 70849 |
| 10 | 3 and 9 | 12747 |
| 11 | (systematic review.ti. or meta-analysis.pt. or meta-analysis.ti. or systematic literature review.ti. or this systematic review.tw. or pooling project.tw. or (systematic review.ti,ab. and review.pt.) or meta synthesis.ti. or meta-analy*.ti. or integrative review.tw. or integrative research review.tw. or rapid review.tw. or umbrella review.tw. or consensus development conference.pt. or practice guideline.pt. or drug class reviews.ti. or cochrane database syst rev.jn. or acp journal club.jn. or health technol assess.jn. or evid rep technol assess summ.jn. or jbi database system rev implement rep.jn. or (clinical guideline and management).tw. or ((evidence based.ti. or evidence-based medicine/ or best practice*.ti. or evidence synthesis.ti,ab.) and (((review.pt. or diseases category/ or behavior.mp.) and behavior mechanisms/) or therapeutics/ or evaluation studies.pt. or validation studies.pt. or guideline.pt. or pmcbook.mp.)) or (((systematic or systematically).tw. or critical.ti,ab. or study selection.tw. or ((predetermined or inclusion) and criteri*).tw. or exclusion criteri*.tw. or main outcome measures.tw. or standard of care.tw. or standards of care.tw.) and ((survey or surveys).ti,ab. or overview*.tw. or review.ti,ab. or reviews.ti,ab. or search*.tw. or handsearch.tw. or analysis.ti. or critique.ti,ab. or appraisal.tw. or (reduction.tw. and (risk/ or risk.tw.) and (death or recurrence).mp.)) and ((literature or articles or publications or publication or bibliography or bibliographies or published).ti,ab. or pooled data.tw. or unpublished.tw. or citation.tw. or citations.tw. or database.ti,ab. or internet.ti,ab. or textbooks.ti,ab. or references.tw. or scales.tw. or papers.tw. or datasets.tw. or trials.ti,ab. or meta-analy*.tw. or (clinical and studies).ti,ab. or treatment outcome/ or treatment outcome.tw. or pmcbook.mp.))) not (letter or newspaper article).pt. | 445481 |
| 12 | 10 and 11 | 481 |
| 13 | limit 12 to english language | 460 |
SYSTEMATIC REVIEW SEARCH: COCHRANE DATABASE OF SYSTEMATIC REVIEWS (10-14-20)
Search for current systematic reviews CDSR Date Searched: 10-14-20 | ||
|---|---|---|
| # | Search Statement | Results: |
| 1 | MeSH descriptor: [Dental Care] explode all trees | 21 |
| 2 | MeSH descriptor: [Dental Care for Chronically Ill] explode all trees | 0 |
| 3 | MeSH descriptor: [Preventive Dentistry] explode all trees | 29 |
| 4 | MeSH descriptor: [Oral Hygiene] explode all trees | 23 |
| 5 | MeSH descriptor: [Oral Health] explode all trees | 15 |
| 6 | MeSH descriptor: [Periodontal Diseases] explode all trees | 32 |
| 7 | MeSH descriptor: [Dental Caries] explode all trees | 41 |
| 8 | (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or oral health or dental hygiene or oral hygiene or periodont* or parodontos* or pyorrhea alveolaris or gingivitis or gingiva* or paradont* or dental decay or dental caries or carious dentin$1 or root decay):ti,ab,kw | 684 |
| 9 | 685 | |
| 10 | MeSH descriptor: [Cardiovascular Diseases] explode all trees | 962 |
| 11 | MeSH descriptor: [Cerebrovascular Disorders] explode all trees | 307 |
| 12 | MeSH descriptor: [Diabetes Mellitus] explode all trees | 205 |
| 13 | MeSH descriptor: [Pulmonary Disease, Chronic Obstructive] explode all trees | 95 |
| 14 | (((cardiovascular or heart) N2 disease$1) or atheroscleros* or arterioscleros*):ti.ab,kw | 46 |
| 15 | ((cerebrovascular N2 (disorder$1 or disease$1 or occlusion$1 or insufficienc*)) or intracranial vascular disease$1 or intracranial vascular disorder$1 or brain vascular disorder$1 or stroke):ti,ab,kw | 517 |
| 16 | (diabetes or diabetic or glycemic control):ti,ab,kw | 434 |
| 17 | (COPD or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic obstructive pulmonary disease or chronic airflow obstruction):ti,ab,kw | 137 |
| 18 | (OR #10-#17) | 1544 |
| 19 | #9 AND #18 | 97 |
PRIMARY STUDY GAP SEARCH: OVID MEDLINE (12-8-20)
Search for primary literature MEDLINE Date searched: 12-08-20 | ||
|---|---|---|
| Search #1 | ||
| # | Search Statement | Results |
| 1 | exp Cerebrovascular Disorders/ or exp Pulmonary Disease, Chronic Obstructive/ or ((cerebrovascular adj2 (disorder$1 or disease$1 or occlusion$1 or insufficienc*)) or intracranial vascular disease$1 or intracranial vascular disorder$1 or brain vascular disorder$1 or stroke or COPD or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic obstructive pulmonary disease or chronic airflow obstruction).ti,ab. | 584995 |
| 2 | exp Dental Care/ or Dental Care for Chronically Ill/ or Preventive Dentistry/ or exp Periodontal Diseases/ or (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 177113 |
| 3 | 1 AND 2 | 948 |
| 4 | Limit 3 to English language | 867 |
| Search #2 | ||
| # | Search Statement | Results |
| 1 | exp Cardiovascular Diseases/ or exp Diabetes Mellitus/ or (((cardiovascular or heart) adj2 disease$1) or atherosclerosis or arteriosclerosis or diabetes or diabetic or glycemic control).ti,ab. | 3069969 |
| 2 | exp Dental Care/ or Dental Care for Chronically Ill/ or Preventive Dentistry/ or exp Periodontal Diseases/ or (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 177600 |
| 3 | Patient Acceptance of Health Care/ or Health Care Costs/ or Quality of Life/ or Self Report/ or ((health?care adj1 (utilization or acceptance or acceptor$1 or seeking or costs$1)) or health cost$1 or treatment cost$1 or (quality adj1 life) or self?report$1 or (patient-reported adj2 symptom$1)).ti,ab. | 338796 |
| 4 | 1 and 2 and 3 | 166 |
| 5 | Limit 4 to English language | 159 |
PRIMARY STUDY GAP SEARCH: CCRCT (12-14-20)
Search for primary literature CCRCT Date searched: 12-14-20 | ||
|---|---|---|
| Search #1 | ||
| # | Search Statement | Results |
| 1 | ((cerebrovascular adj2 (disorder$1 or disease$1 or occlusion$1 or insufficienc*)) or intracranial vascular disease$1 or intracranial vascular disorder$1 or brain vascular disorder$1 or stroke or COPD or COAD or chronic obstructive airway disease or chronic obstructive lung disease or chronic obstructive pulmonary disease or chronic airflow obstruction).ti,ab. | 75984 |
| 2 | (Cerebrovascular Disorders or Pulmonary Disease, Chronic Obstructive).kw. | 85 |
| 3 | 1 OR 2 | 76005 |
| 4 | (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 14433 |
| 5 | (Dental Care or Dental Care for Chronically Ill or Preventive Dentistry or Periodontal Diseases).kw. | 141 |
| 6 | 4 OR 5 | 14482 |
| 7 | 3 AND 6 | 70 |
| 8 | Limit 7 to English language | 45 |
| Search #2 | ||
| # | Search Statement | Results |
| 1 | (((cardiovascular or heart) adj2 disease$1) or atherosclerosis or arteriosclerosis or diabetes or diabetic or glycemic control).ti,ab. | 128047 |
| 2 | (Cardiovascular Disease or Diabetes Mellitus).kw. | 36268 |
| 3 | 1 or 2 | 133483 |
| 4 | (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 14433 |
| 5 | (Dental Care or Dental Care for Chronically Ill or Preventive Dentistry or Periodontal Diseases).kw. | 141 |
| 6 | 4 or 5 | 14482 |
| 7 | ((health?care adj1 (utilization or acceptance or acceptor$1 or seeking or costs$1)) or health cost$1 or treatment cost$1 or (quality adj1 life) or self?report$1 or (patient-reported adj2 symptom$1)).ti,ab. | 108801 |
| 8 | (Patient Acceptance of Health Care or Health Care Costs or Quality of Life or Self Report).kw. | 40851 |
| 9 | 7 or 8 | 119502 |
| 10 | 3 AND 6 AND 9 | 35 |
| 11 | Limit 10 to English language | 15 |
PRIMARY STUDY UPDATE SEARCH: OVID MEDLINE (2-5-21)
Ovid MEDLINE(R) ALL 1946 to February 03, 2021 Date Searched: 02-05-21 | ||
|---|---|---|
| # | Search Statement | Results |
| 1 | exp Cardiovascular Diseases/ or exp Diabetes Mellitus/ or (((cardiovascular or heart) adj2 disease$1) or atherosclerosis or arteriosclerosis or diabetes or diabetic or glycemic control).ti,ab. | 3091462 |
| 2 | exp Dental Care/ or Dental Care for Chronically Ill/ or Preventive Dentistry/ or exp Periodontal Diseases/ or (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 178775 |
| 3 | Chronic Disease Indicators/ or Glycated Hemoglobin A/ or exp Triglycerides/ or C-Reactive Protein/ or Interleukin-6/ or Cholesterol, HDL/ or Cholesterol, LDL/ or (clinical outcome$1 OR chronic disease indicator$1 OR HbA1c OR glycated hemoglobin OR fasting blood glucose OR total cholesterol OR triglycerides OR tricylglycerol$1 OR HDL OR LDL OR C-reactive protein OR IL-6 OR systematic inflammation OR endothelial function OR TNF-alpha OR blood pressure or hs?CRP or interleukin 6 or MGI-2 or cholesterol).ti,ab. | 1118111 |
| 4 | 1 or 2 or 3 | 2048 |
| 5 | Limit 4 to english language and yr=”2019-2020”) | 263 |
PRIMARY STUDY UPDATE SEARCH: CCRCT (2-5-20)
EBM Reviews - Cochrane Central Register of Controlled Trials December 2020 Date Searched: 02-05-21 | ||
|---|---|---|
| # | Search Statement | Results |
| 1 | exp Cardiovascular Diseases/ or exp Diabetes Mellitus/ or (((cardiovascular or heart) adj2 disease$1) or atherosclerosis or arteriosclerosis or diabetes or diabetic or glycemic control).ti,ab. | 216678 |
| 2 | exp Dental Care/ or Dental Care for Chronically Ill/ or Preventive Dentistry/ or exp Periodontal Diseases/ or (dental health service$1 or dental care or dental practice or preventive dentistry or preventive dentistries or periodontal or periodontitis or parodontosis or pyorrhea alveolaris or gingivitis or gingiva or gingival or paradontal or paradontopathia or paradontosis).ti,ab. | 16291 |
| 3 | Chronic Disease Indicators/ or Glycated Hemoglobin A/ or exp Triglycerides/ or C-Reactive Protein/ or Interleukin-6/ or Cholesterol, HDL/ or Cholesterol, LDL/ or (clinical outcome$1 OR chronic disease indicator$1 OR HbA1c OR glycated hemoglobin OR fasting blood glucose OR total cholesterol OR triglycerides OR tricylglycerol$1 OR HDL OR LDL OR C-reactive protein OR IL-6 OR systematic inflammation OR endothelial function OR TNF-alpha OR blood pressure or hs?CRP or interleukin 6 or MGI-2 or cholesterol).ti,ab. | 187811 |
| 4 | 1 or 2 or 3 | 388 |
| 5 | Limit 4 to english language and yr=”2019-2020”) | 23 |
INCLUSION/EXCLUSION CRITERIA
| Include | Exclude | Code | |
|---|---|---|---|
| Population | Include adults with cardiovascular disease, cerebrovascular disease, diabetes, or COPD. | Exclude children; pregnant adults; adults without chronic diseases; adults with chronic diseases other than those listed | E1 |
| Intervention | Include SRs- any preventive dental treatment Primary studies- periodontal treatment | Exclude patient brushing/flossing, treatment of rare or advanced conditions occurring outside the routine dental care setting | E2 |
| Comparator | Include: no detection or treatment of dental problems | Exclude other active comparators such as alternative treatments, alternative timing of treatments, and alternative intensity of treatments | E3 |
| Outcomes | Include
| Exclude all other outcomes | E4 |
| Timing | Include any timing | NA | E5 |
| Setting | Include any setting | NA | E6 |
| Study Design | Include SRs- Any that meet the following 4 criteria-
| Exclude SRs- those that don’t meet these 4 criteria Primary studies- NA | E7 |
| Publication type | Include full-text studies | Exclude: Abstract only, protocol only, editorial, letter, narrative review, guidelines | E8 |
| Outdated or ineligible SR | NA | Exclude systematic reviews that use outdated methods or were updated by another, published review | E9 |
| Language | Include English | Exclude languages other than English | E10 |
LIST OF EXCLUDED STUDIES
Exclude reasons: 1=Ineligible population, 2=Ineligible intervention, 3=Ineligible comparator, 4=Ineligible outcome, 5=Ineligible timing, 6=Ineligible setting, 7=Ineligible study design, 8=Ineligible publication type, 9=Outdated or ineligible systematic review, 10=Not available in English
| # | Citation | Exclude reason |
|---|---|---|
| 1 | Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers in Medical Science. 2017;32(2):449–459. [PubMed: 27686888] | E3 |
| 2 | Alexander RE, Grogan DM. Management of dental patients with obstructive lung diseases. Texas Dental Journal. 2008;125(3):228–240. [PubMed: 18481611] | E8 |
| 3 | Al-Hamoudi N. Is antimicrobial photodynamic therapy an effective treatment for chronic periodontitis in diabetes mellitus and cigarette smokers: a systematic review and meta-analysis. Photodiagnosis & Photodynamic Therapy. 2017;19:375–382. [PubMed: 28559203] | E3 |
| 4 | Alim BA, Canturk E, Koksal C. The effect of treated apical periodontitis before heart valve surgery on C-reactive protein levels. Oral Diseases. 2020;24:24. [PubMed: 32710519] | E1 |
| 5 | Androsz-Kowalska O, Jankowski K, Rymarczyk Z, Kowalski J, Pruszczyk P, Gorska R. Correlation between clinical parameters of periodontal disease and mean platelet volume in patients with coronary artery disease: a pilot study. Kardiologia Polska. 2013;71(6):600–605. [PubMed: 23797433] | E4 |
| 6 | Brignardello-Petersen R. There seem to be no benefits from periodontal treatment on glycated hemoglobin levels of patients with uncontrolled diabetes mellitus 3 months after treatment. Journal of the American Dental Association. 2019;150(4):e43. [PubMed: 30593341] | E7 |
| 7 | Brignardello-Petersen R. There is still no high-quality evidence that periodontitis is a risk factor for hypertension or that periodontal treatment has beneficial effects on blood pressure. Journal of the American Dental Association. 2020;151(4):e31. [PubMed: 32033741] | E7 |
| 8 | Brown TT, Dela Cruz E, Brown SS. The effect of dental care on cardiovascular disease outcomes: an application of instrumental variables in the presence of heterogeneity and self-selection. Health Economics. 2011;20(10):1241–1256. [PubMed: 20882577] | E2 |
| 9 | Chandra S, Shashikumar P. Diode Laser - A Novel Therapeutic Approach in the Treatment of Chronic Periodontitis in Type 2 Diabetes Mellitus Patients: A Prospective Randomized Controlled Clinical Trial. Journal of Lasers in Medical Sciences. 2019;10(1):56–63. [PMC free article: PMC6499582] [PubMed: 31360370] | E7 |
| 10 | Chen ZY, Chiang CH, Huang CC, et al. The association of tooth scaling and decreased cardiovascular disease: a nationwide population-based study. American Journal of Medicine. 2012;125(6):568–575. [PubMed: 22483056] | E1 |
| 11 | Cortelli SC, Costa FO, Gargioni-Filho A, et al. Impact of gingivitis treatment for diabetic patients on quality of life related to periodontal objective parameters: A randomized controlled clinical trial. Archives of Oral Biology. 2018;86:80–86. [PubMed: 29197785] | E2 |
| 12 | Cotti E, Arrica M, Di Lenarda A, et al. The perioperative dental screening and management of patients undergoing cardiothoracic, vascular surgery and other cardiovascular invasive procedures: A systematic review. European Journal of Preventive Cardiology. 2017;24(4):409–425. [PubMed: 28094561] | E7 |
| 13 | Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. European Heart Journal. 2019;40(42):3459–3470. [PMC free article: PMC6837161] [PubMed: 31504461] | E3 |
| 14 | D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. Journal of Periodontology. 2013;84:(4 Suppl):S85–S105. [PubMed: 23631587] | E7 |
| 15 | Darre L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes & Metabolism. 2008;34(5):497–506. [PubMed: 18948050] | E7 |
| 16 | de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ. 2010;340:c2451. [PMC free article: PMC2877809] [PubMed: 20508025] | E2 |
| 17 | Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. Journal of Periodontology. 2013;84:(4 Suppl):S153–169. [PMC free article: PMC4100543] [PubMed: 23631575] | E7 |
| 18 | Freitas CO, Gomes-Filho IS, Naves RC, et al. Influence of periodontal therapy on C-reactive protein level: a systematic review and meta-analysis. Journal of Applied Oral Science. 2012;20(1):1–8. [PMC free article: PMC3928764] [PubMed: 22437670] | E1 |
| 19 | Garcia R. Periodontal treatment could improve glycaemic control in diabetic patients. Evidence-Based Dentistry. 2009;10(1):20–21 [PubMed: 19322226] | E8 |
| 20 | Garg A, Guez G. Study links oral care to lower diabetes costs. Dental Implantology Update. 2012;23(5):37–40. [PubMed: 22606706] | E8 |
| 21 | Henschel M, Keenan AV. Insufficient evidence of effect of periodontal treatment on prevention or management of cardiovascular disease. Evidence-Based Dentistry. 2015;16(1):17–18. [PubMed: 25909935] | E8 |
| 22 | Huang JL, Chen WK, Lin CL, et al. Association between intensive periodontal treatment and spontaneous intracerebral hemorrhage-a nationwide, population-based cohort study. Medicine. 2019;98(10):e14814. [PMC free article: PMC6417639] [PubMed: 30855503] | E1 |
| 23 | Improved Health and Lower Medical Costs: Why Good Dental Care is Important. Cigna. https://www | E7 |
| 24 | Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. J Periodontol. 2006 Oct;77(10):1635–42. doi: 10.1902/jop.2006.050443. PMID: 17032104. [PubMed: 17032104] [CrossRef] | E1 |
| 25 | Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. Journal of Dentistry. 2015;43(5):506–511. [PubMed: 25769264] | E3 |
| 26 | Janket SJ. Scaling and root-planing (SRP) may improve glycemic control and lipid profile in patients with chronic periodontitis (CP) and type 2 diabetes (DM2) in a specific subgroup: a meta-analysis of randomized clinical trials. The Journal of Evidencebased Dental Practice. 2014;14(1):31–33 [PubMed: 24581710] | E8 |
| 27 | Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. Journal of Dental Research. 2005;84(12):1154–1159. [PMC free article: PMC1797067] [PubMed: 16304446] | E7 |
| 28 | Jeffcoat M, Hall M, Hedlund C. Periodontal treatment and medical costs in diabetes and cerebrovascular accident. Paper presented at: International Association for Dental Research Meeting, Miami, Florida2009. | E8 |
| 29 | Jones JA, Miller DR, Wehler CJ, et al. Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. Journal of Clinical Periodontology. 2007;34(1):46–52. [PubMed: 17137468] | E4 |
| 30 | Jones JA, Miller DR, Wehler CJ, et al. Study design, recruitment, and baseline characteristics: the Department of Veterans Affairs Dental Diabetes Study. Journal of Clinical Periodontology. 2007;34(1):40–45. [PubMed: 17040483] | E4 |
| 31 | Kim HK, Kim YG, Cho JH, Lee SK, Lee JM. The effect of periodontal and prosthodontic therapy on glycemic control in patients with diabetes. The Journal of Advanced Prosthodontics. 2019;11(5):247–252. [PMC free article: PMC6856313] [PubMed: 31754414] | E3 |
| 32 | Kumar G, Ponnaiyan D, Parthasarathy H, Tadepalli A, Veeramani S. Evaluation of Endocan and Tumor Necrosis Factor-alpha as Inflammatory Biomarkers in Type 2 Diabetes and Periodontal Disease. Genetic Testing & Molecular Biomarkers. 2020;24(7):431–435. [PubMed: 32513032] | E8 |
| 33 | Lam OL, Zhang W, Samaranayake LP, Li LS, McGrath C. A systematic review of the effectiveness of oral health promotion activities among patients with cardiovascular disease. International Journal of Cardiology. 2011;151(3):261–267. [PubMed: 21176980] | E7 |
| 34 | Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database of Systematic Reviews. 2014(8):CD009197. [PubMed: 25123257] | E9 |
| 35 | Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database of Systematic Reviews. 2017;11:CD009197. [PMC free article: PMC6486158] [PubMed: 29112241] | E9 |
| 36 | Lin HW, Chen CM, Yeh YC, et al. Dental treatment procedures for periodontal disease and the subsequent risk of ischaemic stroke: A retrospective population-based cohort study. Journal of Clinical Periodontology. 2019;46(6):642–649. [PubMed: 30989681] | E1 |
| 37 | Luo H, Bell RA, Wright W, Wu Q, Wu B. Trends in annual dental visits among US dentate adults with and without self-reported diabetes and prediabetes, 2004-2014. Journal of the American Dental Association. 2018;149(6):460–469 [PubMed: 29615188] | E2 |
| 38 | Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. Journal of Clinical Periodontology. 2018;45(2):188–195. [PubMed: 29277978] | E7 |
| 39 | Marano
A, Hahn
M, Hall
M, Hedlund
C, Sun
C, R G. Appropriate Periodontal Therapy Associated with Lower Medical Utilization and Costs. Cigna. https://www | E8 |
| 40 | Merchant AT. Will periodontal treatment prevent heart disease and stroke? The Journal of Evidencebased Dental Practice. 2012;12(4):212–215. [PubMed: 23177502] | E8 |
| 41 | Mirza S, Khan AA, Al-Kheraif AA, Khan SZ, Shafqat SS. Efficacy of adjunctive photodynamic therapy on the clinical periodontal, HbA1c and advanced glycation end product levels among mild to moderate chronic periodontal disease patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Photodiagnosis & Photodynamic Therapy. 2019;28:177–182. [PubMed: 31394300] | E3 |
| 42 | Montenegro MM, Ribeiro IWJ, Kampits C, et al. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. Journal of Clinical Periodontology. 2019;46(3):321–331. [PubMed: 30761568] | E3 |
| 43 | Mori C, Hakuta C, Endo K, et al. The effects of professional oral health care on patients in the subacute stage of emergent neurosurgical disorders. Special Care in Dentistry. 2012;32(6):259–264. [PubMed: 23095069] | E1 |
| 44 | Moura Foz A, Alexandre Romito G, Manoel Bispo C, et al. Periodontal therapy and biomarkers related to cardiovascular risk. Minerva Stomatologica. 2010;59(5):271–283. [PubMed: 20502435] | E7 |
| 45 | Munjal A, Jain Y, Kote S, et al. A study on the change in HbA1c levels before and after non-surgical periodontal therapy in type-2 diabetes mellitus in generalized periodontitis. Journal of Family Medicine & Primary Care. 2019;8(4):1326–1329. [PMC free article: PMC6510074] [PubMed: 31143715] | E3 |
| 46 | Munoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovascular Research. 2020;116(1):28–39. [PubMed: 31549149] | E7 |
| 47 | Nishioka S, Maruyama K, Tanigawa T, et al. Effect of non-surgical periodontal therapy on insulin resistance and insulin sensitivity among individuals with borderline diabetes: A randomized controlled trial. Journal of Dentistry. 2019;85:18–24. [PubMed: 30986513] | E1 |
| 48 | Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clinical Oral Implants Research. 2013;24(2):117–127. [PMC free article: PMC3329564] [PubMed: 22111901] | E7 |
| 49 | Obadan-Udoh E, Jordan S, Mudah O, Borgnakke W, Tavares M. Gap Analysis of Older Adults With Type 2 Diabetes Receiving Nonsurgical Periodontal Therapy. The Journal of Evidencebased Dental Practice. 2017;17(4):335–349. [PubMed: 29197435] | E7 |
| 50 | Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. [PubMed: 25014033] | E7 |
| 51 | Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clinical Oral Implants Research. 2013;24(2):117–127. [PMC free article: PMC3329564] [PubMed: 22111901] | E7 |
| 52 | Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. Journal of Clinical Periodontology. 2008;35(4):277–290. [PubMed: 18294231] | E1 |
| 53 | Patil VA, Desai MH. Effect of periodontal therapy on serum C-reactive protein levels in patients with gingivitis and chronic periodontitis: a clinicobiochemical study. Journal of Contemporary Dental Practice [Electronic Resource]. 2013;14(2):233–237 [PubMed: 23811651] | E1 |
| 54 | Parenti A, Paccosi S, Cairo F, Defraia E. Treatment of Periodontitis for the Prevention of Endothelial Dysfunction: A Narrative Review. Current Vascular Pharmacology. 2015;13(6):749–758. [PubMed: 26282351] | E7 |
| 55 | Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. European Heart Journal. 2019;40(14):1138–1145. [PubMed: 30561631] | E2 |
| 56 | Pedersen PU, Uhrenfeldt L, Larsen P. Oral hygiene in patients with chronic obstructive pulmonary disease: a scoping review protocol. JBI Database Of Systematic Reviews And Implementation Reports. 2017;15(5):1236–1241. [PubMed: 28498163] | E2 |
| 57 | Polzer I, Schwahn C, Volzke H, Mundt T, Biffar R. The association of tooth loss with all-cause and circulatory mortality. Is there a benefit of replaced teeth? A systematic review and meta-analysis. Clinical Oral Investigations. 2012;16(2):333–351. [PubMed: 22086361] | E1 |
| 58 | Pohjamo L, Tervonen T, Knuuttila M, Nurkkala H. Adult diabetic and nondiabetic subjects as users of dental services. A longitudinal study. Acta Odontologica Scandinavica. 1995;53(2):112–114. [PubMed: 7610774] | E3 |
| 59 | Ramirez JH, Arce RM, Contreras A. Periodontal treatment effects on endothelial function and cardiovascular disease biomarkers in subjects with chronic periodontitis: protocol for a randomized clinical trial. Trials [Electronic Resource]. 2011;12:46. [PMC free article: PMC3049125] [PubMed: 21324167] | E4 |
| 60 | Redd KT, Phillips ST, McMillian B, et al. PeRiodontal Treatment to Eliminate Minority Inequality and Rural Disparities in Stroke (PREMIERS): A Multicenter, Randomized, Controlled Study. International Journal of Cerebrovascular Disease & Stroke. 2019;2(2). [PMC free article: PMC7064156] [PubMed: 32159164] | E4 |
| 61 | Reichert S, Schlitt A, Beschow V, et al. Use of floss/interdental brushes is associated with lower risk for new cardiovascular events among patients with coronary heart disease. Journal of Periodontal Research. 2015;50(2):180–188. [PubMed: 24824149] | E2 |
| 62 | Roca-Millan E, Gonzalez-Navarro B, Sabater-Recolons MM, Mari-Roig A, Jane-Salas E, Lopez-Lopez J. Periodontal treatment on patients with cardiovascular disease: Systematic review and meta-analysis. Medicina Oral, Patologia Oral y Cirugia Bucal. 2018;23(6):e681–e690. [PMC free article: PMC6261003] [PubMed: 30341272] | E7 |
| 63 | Romero SS, Pinto EH, Longo PL, et al. Effects of periodontal treatment on exacerbation frequency and lung function in patients with chronic periodontitis: study protocol of a 1-year randomized controlled trial. BMC Pulmonary Medicine. 2017;17(1):23. [PMC free article: PMC5259840] [PubMed: 28114928] | E4 |
| 64 | Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. Journal of Clinical Periodontology. 2008;35:(8 Suppl):398–409. [PubMed: 18724865] | E7 |
| 65 | Sanz M, Ceriello A, Buysschaert M, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Journal of Clinical Periodontology. 2018;45(2):138–149. [PubMed: 29280174] | E8 |
| 66 | Sanchez P, Salamonson Y, Everett B, George A. Barriers and Predictors Associated With Accessing Oral Healthcare Among Patients With Cardiovascular Disease in Australia. Journal of Cardiovascular Nursing. 2019;34(3):208–214. [PubMed: 30589656] | E4 |
| 67 | Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. American Heart Journal. 2005;149(6):1050–1054. [PubMed: 15976787] | E1 |
| 68 | Sen S, Giamberardino LD, Moss K, et al. Periodontal Disease, Regular Dental Care Use, and Incident Ischemic Stroke. Stroke. 2018;49(2):355–362. [PMC free article: PMC5780242] [PubMed: 29335336] | E1 |
| 69 | Seshima F, Nishina M, Namba T, Saito A. Periodontal Regenerative Therapy in Patient with Chronic Periodontitis and Type 2 Diabetes Mellitus: A Case Report. Bulletin of Tokyo Dental College. 2016;57(2):97–104. [PubMed: 27320299] | E3 |
| 70 | Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. Journal of Periodontology. 2013;84(7):958–973. [PubMed: 23106512] | E9 |
| 71 | Shen TC, Chang PY, Lin CL, et al. Periodontal Treatment Reduces Risk of Adverse Respiratory Events in Patients With Chronic Obstructive Pulmonary Disease: A Propensity-Matched Cohort Study. Medicine. 2016;95(20):e3735. [PMC free article: PMC4902439] [PubMed: 27196497] | E3 |
| 72 | Sheiham A. Claims that periodontal treatment reduces costs of treating five systemic conditions are questionable. The Journal of Evidencebased Dental Practice. 2015;15(1):35–36. [PubMed: 25666581] | E8 |
| 73 | Skaar D, O’Connor H, Lunos S, Luepker R, Michalowicz BS. Dental procedures and risk of experiencing a second vascular event in a Medicare population. Journal of the American Dental Association. 2012;143(11):1190–1198 [PubMed: 23115147] | E2 |
| 74 | Sun WL, Chen LL, Zhang SZ, Ren YZ, Qin GM. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Archives of Oral Biology. 2010;55(12):970–974. [PubMed: 20889139] | E4 |
| 75 | Syrjala AM, Kneckt MC, Knuuttila ML. Dental self-efficacy as a determinant to oral health behaviour, oral hygiene and HbA1c level among diabetic patients. Journal of Clinical Periodontology. 1999;26(9):616–621. [PubMed: 10487313] | E2 |
| 76 | Taylor GW. Periodontal treatment and its effects on glycemic control: a review of the evidence. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics. 1999;87(3):311–316. [PubMed: 10102591] | E7 |
| 77 | Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Annals of Periodontology. 2001;6(1):99–112. [PubMed: 11887478] | E7 |
| 78 | Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):421–427. [PMC free article: PMC2809296] [PubMed: 20103557] | E1 |
| 79 | Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. Journal of Clinical Periodontology. 2009;36: Suppl 10:15–19. [PubMed: 19432627] | E6 |
| 80 | Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. [PubMed: 17329698] | E1 |
| 81 | Vergnes JN. Treating periodontal disease may improve metabolic control in diabetics. Evidence-Based Dentistry. 2010;11(3):73–74 [PubMed: 20938470] | E8 |
| 82 | Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2014;9(9):e108412. [PMC free article: PMC4177914] [PubMed: 25255331] | E2 |
| 83 | Watts T. Periodontal treatment and glycemic control in diabetic patients: the problem of a possible Hawthorne effect. Journal of Dental Research. 2006;85(4):294; author reply 294–295. [PubMed: 16567546] | E8 |
SEVENTEEN INCLUDED BUT DE-PRIORITIZED SYSTEMATIC REVIEWS
| Author Year | Reason de-prioritized |
|---|---|
| Cao 20191 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Jain 20192 | Included in Ata-Ali 2020 |
| Hasuike 20173 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Manger 20174 | Did not find any studies on link between periodontal disease treatment & COPD |
| Botero 20165 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Faggion 20166 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Perez Losado 20167 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Teshome 20168 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Artese 20159 | PICOs covered by more recent reviews (systematic inflammation: Beza 2020 & Lima 2019) or more comprehensive review (harms: Simpson 2015). |
| Li 201510 | Included in Ata-Ali 2020 |
| Mauri-Obradors 201511 | Included in Ata-Ali 2020 |
| Schmitt 201512 | Only potentially relevant outcome measured among people with hypertension was “arterial stiffness” which was evaluated in a single study and has limited clinical relevance. |
| Sun 201413 | Included in Ata-Ali 2020 |
| Wang 201414 | Included in Ata-Ali 2020 |
| Corbella 201315 | PICOs covered by more recent review of reviews (Ata-Ali 2020) |
| Liew 201316 | Included in Ata-Ali 2020 |
| Teeuw 201017 | Included in Ata-Ali 2020 |
EVIDENCE TABLES
DATA ABSTRACTION OF PRIORITIZED SYSTEMATIC REVIEWS
Download PDF (304K)
DATA ABSTRACTION OF INCLUDED PRIMARY STUDIES
Download PDF (378K)
QUALITY ASSESSMENT OF PRIORITIZED SYSTEMATIC REVIEWS
Quality Assessment of Prioritized Systematic Reviews (PDF, 209K)
QUALITY ASSESSMENT OF INCLUDED PRIMARY STUDIES
Quality Assessment of RCTs (PDF, 216K)
Quality Assessment of Controlled Observational Studies (PDF, 261K)
PEER REVIEW COMMENT TABLE
| Comment # | Reviewer # | Comment | Author Response |
|---|---|---|---|
| Are the objectives, scope, and methods for this review clearly described? | |||
| 1 | 1 | Yes | None |
| 2 | 2 | No - This may be a function of the form prescribed by the VA for Evidence Synthesis documents, but I find the document redundant is places, and therefore difficult to read and interpret. A simpler and more streamlined style should be considered. | Thank you for this comment. We have streamlined the “Results” section to make the report more reader-friendly. For each population, we collapsed the separate sections on “systematic reviews” and “primary studies” into a single section. We have also combined the 2 separate tables on systematic review and primary study findings into a single table. |
| 3 | 3 | Yes | None |
| 4 | 4 | Yes | None |
| Is there any indication of bias in our synthesis of the evidence? | |||
| 4 | 1 | No | None |
| 5 | 2 | No | None |
| 6 | 3 | No | None |
| 7 | 4 | Yes - The authors’ explanation for excluding relevant studies were not satisfactory. The need to conduct a rapid synthesis does not preclude the authors from considering all available and relevant evidence. Since this approach already skipped some steps by focusing only on gaps that were found in existing systematic reviews and some systematic reviews are a few years old, it seems reasonable to expect the authors to have at least included all the relevant publications that they found. | For the 17 SRs we included but did not prioritize, we have added a brief explanation of our prioritization rationale to the Results section. Most often, these SRs were not prioritized because they were already included in a prioritized review of reviews (Ata-Ali 2020), or their PICOS were covered by a more recent or more relevant review. For transparency, we have also added a table to the Supplementary Materials to provide a list of the 17 non-prioritized SRs along with a specific rationale for why each was not discussed in the report. For primary studies, we included all studies that addressed gaps in evidence from our included SRs (we did not prioritize any primary studies over others – all were discussed). We have added a sentence to the methods section to clarify this. |
| Are there any published or unpublished studies that we may have overlooked? | |||
| 8 | 1 | Yes - A number of economic studies have been conducted by insurers but are not listed. See below: | See responses to comments #9-14. |
| 9 | 1 | Cigna. Improved Health and Lower Medical Costs: Why Good Dental Care is Important. 2010. https://www | Excluded- wrong publication type. Though this white paper presents some data on our PICOS of interest, there is not enough information on the methods or results to include this as a study. |
| 10 | 1 | Jeffcoat, M. K., et al. 2009. “Periodontal Treatment and Medical Costs in Diabetes and Cerebrovascular Accident.” Paper presented at the 2009 International Association for Dental Research Meeting, Miami, Florida. J | Excluded- wrong publication type. We could not track down the abstract (it is no longer available on the conference website) or FT article for this study. |
| 11 | 1 | Jeffcoat MK, Jeffcoat RL, Gladowski PA, et al. Impact of periodontal therapy on general health: Evidence from insurance data for five systemic conditions. Am J Prev Med. 2014;47(2):166–74 [PubMed: 24953519]. | This study was included in our report. |
| 12 | 1 | Marano, A., et al. 2013. Appropriate Periodontal Therapy Associated with Lower Medical Utilization and Costs. Bloomfield, CT: Cigna | Excluded- wrong publication type. We could not track down the FT for this study. However, based on the abstract, this study does not seem to be focused on people with chronic conditions. |
| 13 | 1 | Nasseh
K, Vujicic
M, Glick
M. The relationship between periodontal interventions and healthcare costs and utilization: Evidence from an integrated dental, medical, and pharmacy commercial claims database. Health Econ. 2017;26:519–527. http: | We have added this study to the report. |
| 14 | 1 | United Healthcare. Medical Dental Integration Study
2013. http://www | We have added this study to the report. |
| 15 | 2 | Yes - See the comments below for published papers that were not included | See responses to comments #32-33. |
| 16 | 3 | No | None |
| 17 | 4 | Yes - In addition to re-considering all the relevant studies from the list of excluded studies, a quick pubmed search showed some potentially relevant studies that the authors did not exclude: | See response to comment #7 regarding how we prioritized SRs for discussion, and a brief overview of how we included all relevant studies that addressed gaps in SR evidence. See responses to comments #18-27 regarding your additional suggested articles. |
| 18 | 4 | Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019 Aug 6;19(1):176. doi: 10.1186/s12903-019-0829-y. PMID: 31387569; PMCID: PMC6685286 [PMC free article: PMC6685286] [PubMed: 31387569] [CrossRef] | This SR was included in our report but de-prioritized because Ata-Ali 2020 covered the same PICOS and was more recent. We have added a table to the Supplementary Materials to provide a list of the de-prioritized SRs and the reasons they were not discussed. |
| 19 | 4 | Al-Hamoudi N. Is antimicrobial photodynamic therapy an effective treatment for chronic periodontitis in diabetes mellitus and cigarette smokers: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2017 Sep;19:375–382. doi: 10.1016/j.pdpdt.2017.05.018. Epub 2017 May 27. PMID: 28559203 [PubMed: 28559203] [CrossRef]. | Excluded- wrong comparator. This SR evaluates antimicrobial photodynamic therapy as an adjunct to scaling & root planing- so there is no comparison to “no treatment.” |
| 20 | 4 | Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z. Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci. 2017 Feb;32(2):449–459. doi: 10.1007/s10103-016-2086-5. Epub 2016 Sep 29. PMID: 27686888 [PubMed: 27686888] [CrossRef]. | Excluded- wrong comparator. This SR evaluates laser therapy as an adjunct to scaling & root planing- so there is no comparison to “no treatment.” |
| 21 | 4 | Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016 Jul 30;17(1):31. doi: 10.1186/s12903-016-0249-1. PMID: 27473177; PMCID: PMC4967318 [PMC free article: PMC4967318] [PubMed: 27473177] [CrossRef]. | This SR was included in our report but de-prioritized because Ata-Ali 2020 covered the same PICOS and was more recent. We have added a table to the Supplementary Materials to provide a list of the de-prioritized SRs and the reasons they were not discussed. |
| 22 | 4 | Pérez-Losada FL, Jané-Salas E, Sabater-Recolons MM, Estrugo-Devesa A, Segura-Egea JJ, López-López J. Correlation between periodontal disease management and metabolic control of type 2 diabetes mellitus. A systematic literature review. Med Oral Patol Oral Cir Bucal. 2016 Jul 1;21(4):e440–6. doi: 10.4317/medoral.21048. PMID: 26827070; PMCID: PMC4920457 [PMC free article: PMC4920457] [PubMed: 26827070] [CrossRef]. | This SR was included in our report but de-prioritized because Ata-Ali 2020 covered the same PICOS and was more recent. We have added a table to the Supplementary Materials to provide a list of the de-prioritized SRs and the reasons they were not discussed. |
| 23 | 4 | Freias CO, Gomes-Filho IS, Naves RC, Nogueira Filho Gda R, Cruz SS, Santos CA, Dunningham L, Miranda LF, Barbosa MD. Influence of periodontal therapy on C-reactive protein level: a systematic review and meta-analysis. J Appl Oral Sci. 2012 Feb;20(1):1–8. doi: 10.1590/s1678-77572012000100002. PMID: 22437670; PMCID: PMC3928764 [PMC free article: PMC3928764] [PubMed: 22437670] [CrossRef]. | Excluded- wrong population. Did not evaluate people with chronic diseases. |
| 24 | 4 | Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. J Periodontol. 2006 Oct;77(10):1635–42. doi: 10.1902/jop.2006.050443. PMID: 17032104 [PubMed: 17032104] [CrossRef]. | Excluded- wrong population. Did not evaluate people with chronic diseases. |
| 25 | 4 | Choi SE, Sima C, Pandya A. Impact of Treating Oral Disease on Preventing Vascular Diseases: A Model-Based Cost-effectiveness Analysis of Periodontal Treatment Among Patients With Type 2 Diabetes. Diabetes Care. 2020 Mar;43(3):563–571. doi: 10.2337/dc19-1201. Epub 2019 Dec 27. PMID: 31882408 [PubMed: 31882408] [CrossRef]. | We have added this study to the report. |
| 26 | 4 | Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: Evidence from a German claims database. Diabetes Res Clin Pract. 2020 Dec 24;172:108641. doi: 10.1016/j.diabres.2020.108641. Epub ahead of print. PMID: 33359573 [PubMed: 33359573] [CrossRef]. | We have added this study to the report. |
| 27 | 4 | Nasseh K, Vujicic M, Glick M. The Relationship between Periodontal Interventions and Healthcare Costs and Utilization. Evidence from an Integrated Dental, Medical, and Pharmacy Commercial Claims Database. Health Econ. 2017 Apr;26(4):519–527. doi: 10.1002/hec.3316. Epub 2016 Jan 22. PMID: 26799518; PMCID: PMC5347922 [PMC free article: PMC5347922] [PubMed: 26799518] [CrossRef]. | We have added this study to the report (see comment #13). |
| Additional suggestions or comments can be provided below. If applicable, please indicate the page and line numbers from the draft report. | |||
| 28 | 1 | Well constructed, well written review. | Thank you |
| 29 | 1 | Key findings p. 5 clear | Thank you |
| 30 | 1 | Note: Conceptually, periodontal health is achieved through dx, initial treatment, and ONGOING periodontal care (every 3-6 months). Therefore, the optimal effect may be missed by included studies. PERIODIC, RECURRING treatment is key to periodontal maintenance, thus studies with beneficial effects for 3-6 months is probably the most we can get unless the treatment is continued long term. | We have added 2 sentences to the “Discussion” section to indicate why changes to chronic disease indicators may only be short term: “This may be due to the fact that periodontal treatment is meant to be a continuous preventive intervention (ie, scaling and root planing followed by routine check-ups and addressing subsequent problems that arise). Therefore, a single periodontal treatment session or group of sessions may not be sufficient to improve chronic disease indicators long-term.” |
| 31 | 2 | Thank you for asking me to comment on this Evidence Synthesis document. The literature in is this still-emerging field is not easily summarized, due to the lack of long-term studies and heterogeneity of study design. Nevertheless, the intriguing potential of this body of work, specifically improved health outcomes for patients with certain chronic diseases associated with the provision of preventive dental/periodontal care, makes this an important area of interprofessional research. Here are my comments: | None |
| 32 | 2 | 1. Certain statements in the ‘Key Findings’ are problematic. While the data suggest that preventive care is associated with improved lung function and reduced frequency of exacerbations, it is stated that it is not clear if these improvements translate into reductions in health care utilization and costs. Certainly the first sentence implies reduced health care utilization. As stated in the Summary and Discussion, this phrase appears to be based on one short-term study. a amore specific statement is needed. As for CVD, a report by Tonetti et al (NEJM 2007 PMID 17329698), as well as other literature, has noted improvement in endothelial cell function associated with periodontal treatment. These findings provide additional supporting evidence that are at least as important as the reduction in inflammatory cytokines that are mentioned. | We revised the key findings to include the evidence supporting specific statements. Given one of our newly included studies reported those with COPD who receive periodontal treatment have lower medical costs than those who don’t receive treatment, we removed the clause about healthcare utilization and costs. While we agree endothelial function is an important outcome, the Tonetti study you cite was not conducted in those with chronic diseases, and none of our other included studies report this outcome. One of our newly included studies does measure oxidative stress, so this is now included in the report. |
| 33 | 2 | 2. The evidence of an association of diabetes and periodontitis/periodontal treatment is the strongest of the 4 chronic diseases included in this review. One area of investigation that is of interest is research demonstrating reduced health utilization and costs associated with preventive dental care. These studies utilize existing insurance databases and the first of the published studies (Jeffcoat M et al (Am J Prev Med 2014 PMID 24953519; ref #57) examined four chronic diseases, but has been criticized based on methodological concerns (Sheiham A, J Evid Based Dent Pract 2015 PMID 25666581). Other studies on this subject have focused on diabetes, and the Smits study is cited (ref # 52). Further, another study specifically focused on diabetes is not cited (Nasseh K et al 2017 Health Econ PMID 26799518). In general, for all such studies a major problem is the failure to account for confounding variables. This can be due to a variety of reasons, including the use of existing insurance databases that do not contain all relevant variables. | We have added Nasseh 2017 to the report. We previously discussed the limitations of Jeffcoat 2014 (ref #57) – including the fact that their control group consisted of patients who have had 1, 2, or 3 periodontal treatment visits (not 0 visits), which is one of the major criticisms raised by Sheiham 2015. Additionally, in our “Limitations” section, we previously commented that a major limitation of our included non-RCTs is the “high risk that confounders could have influenced results.” |
| 34 | 2 | 3. The inability to access the grey literature is mentioned, yet perhaps further elaboration is needed for completeness. There is a significant amount of grey literature (‘reports”) in the area of preventive dental services and improved health outcomes. These have been prepared by insurance companies as well as in one case by a health care consulting company, funded by a dental service organization. Interesting to note that some insurance companies have decided to offer expanded preventive dental benefits to enrollees with certain chromic diseases (i.e. diabetes, CVD). | We have reviewed potentially relevant grey literature from insurance care companies suggested by reviewer #1 (see comments #9, #12 & #14) and included studies when they met inclusion criteria. |
| 35 | 2 | 4. No mention is made in the report of specific periodontal bacteria that have been shown to play a role in these associations. Porphyromonas gingivalis is one bacterium that has been widely implicated in these associations, and presents with a range of virulence factors that should be discussed when mechanisms are reviewed. | We have added porphyromonas gingivalis as an example of bacteria that may be involved in the relation between periodontal disease and chronic diseases to the “Background” section. |
| 36 | 4 | General Comments: Overall, I found the study very focused and interesting to read. I believe that the authors were very clear about what they intended to accomplish and laid out their rationale for the study in a succinct manner. They also clearly acknowledged the limitations of the study. | Thank you |
| 37 | 4 | Specific Comments: Key Findings/ Executive Summary: My first impression of the key findings box was that the authors appeared equivocal/ambiguous in their conclusions. The use of words/phrases such as “unclear”, “may improve” repeatedly, connotes a lack of confidence about the findings or their conclusions. Could it be that the results are unclear because of the rapid approach to synthesizing the evidence? My fear is that this uncertainty will leave a similar impression on the policy makers who are the intended audience for this report. The review of the evidence should help the readers lean one way or the other, otherwise it does not add much value to the body of knowledge or the decision-making process. | We have removed the ambiguous language when it was used inappropriately (ie, for findings based on moderate or high-quality strength of evidence). However, we left in this language when we made statements based on low or insufficient strength of evidence. It is important that readers have a sense of our level of confidence based on how we frame our results. To that end, we have added information on the underlying studies supporting specific statements so readers can see why we had low levels of certainty. |
| 38 | 4 | Introduction/ Background: I found it sufficient. The purpose, scope, key questions, and eligibility criteria were clearly laid out. Some minor suggestions: Ln 53, Page 2: Please add a reference about who made this hypothesis. Figure 2: KQ4 should include the treatment arm, for those who received treatment for their dental problems | Thank you. Regarding p. 2, line 53- it is difficult to track down who first made this hypothesis, so instead we’ve rephrased the sentence to say periodontal disease “may contribute to worsening of diseases whose etiology or severity are in part driven by chronic inflammation” and provided 2 references that discuss this possible relationship. Regarding figure 2- we have revised the figure so that KQ4 encompasses the treatment arm as well. |
| 39 | 4 | Methods: Ln 9, Page 7: Spell out acronyms in full at first mention. e.g CDSR | Added full name of CDSR (Cochrane Database of Systematic Reviews). |
| 40 | 4 | Ln 20, Page 7: Why did the authors choose to limit the search of primary studies to only address the gaps found with the systematic reviews? Since some of the systematic reviews are at least a few years old, it would have made for a more robust report if all recent primary studies were included in the synthesis. It would have also helped to have a quantitative synthesis/ meta-analysis for the conditions where no systematic reviews were found. i.e. COPD and cerebrovascular disease. | Ultimately, we chose to only search for primary studies that addressed gaps in SR evidence due to 2 factors- 1) there were several moderate and high-quality SRs that addressed several of our PICOs of interest and 2) the review was a rapid product and was to be completed in a short time frame. However, in response to this comment, we have added a search for primary studies on CVD/diabetes & chronic disease indicators from 2019 – 2020. This will ensure we’ve captured studies published since the end date of our most recent, relevant SRs’ searches. To your second point about meta-analysis- we added a statement in the “synthesis of data” section to explain that we did not conduct a meta-analysis specifically among those with COPD or cerebrovascular disease because of variability in study designs, outcome measurements, and timing of outcome timing. |
| 41 | 4 | Ln 44, Page 7: Please provide a detailed description of the “checking” and consensus process throughout the report. Did the second reviewer review a sample of the initial reviewer’s list, the entire selection, or just the ones where there were disagreements? | We have clarified that all titles, abstract and full text articles were reviewed by one investigator and checked by another. |
| 42 | 4 | Ln 50, Page 7: What was the rationale for prioritizing recent studies? Why did the authors not consider all relevant studies that met their criteria? If the time period was important, then why wasn’t it included in the eligibility criteria? What was the time cutoff point used to determine if a study was recent? It isn’t clearly stated. | Our prioritization process only applied to SRs. We did not have a strict cut-off point to determine what was a “recent” review. Instead, we started with the most recently published reviews and moved backwards in time to see if older reviews’ PICOs were already covered by more recent reviews, or if they offered unique information that should be discussed in the report. We added a sentence to the “results” section to describe the reasons why we did not discuss those 17 non-prioritized SRs, and also added a table to the Supplementary Materials with a specific reason why each SR was not prioritized. |
| 43 | 4 | Results: I strongly feel that the ambiguity of the results are due to the limitations in the approach. An exhaustive search of primary studies for each of these topics, might have yielded more evidence that would have helped to increase the certainty of the authors’ conclusions. By focusing on the gaps found with the systematic reviews and not updating the systematic reviews with findings from more recent studies, the authors might have limited their own ability to make more definitive statements. | Given the availability of multiple, moderate or high-quality SRs on the effect of periodontal therapy on chronic disease indicators for those with CVD and diabetes, we believe it was appropriate to focus our report on those SRs’ results and only run a search for studies on gaps in evidence. However, in response to this and earlier comments, we have added a search for primary studies on the effect of periodontal therapy on chronic disease outcomes for those with CVD or diabetes published from 2019 – 2020 to ensure we’ve captured all the most recently published studies on this topic. |
| 44 | 4 | List of Excluded Studies: I recommend reconsidering some of these studies for inclusion in the synthesis. It is unclear what criteria was used to determine “E5”, “E7”, and “E8”. “R” and “E9” are not defined in the legend. | Thank you for pointing this out- the study labeled “R” was an include and was erroneously added in the excluded table. E9 (outdated or ineligible review) has been added to the table. We have also added our full inclusion/exclusion criteria as an additional appendix in the Supplementary Materials for full transparency. |
REFERENCES
- 1.
- Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health. 2019;19(1):176. [PMC free article: PMC6685286] [PubMed: 31387569]
- 2.
- Jain A, Gupta J, Bansal D, Sood S, Gupta S, Jain A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis. Journal of Indian Society of Periodontology. 2019;23(4):303–310. [PMC free article: PMC6628769] [PubMed: 31367125]
- 3.
- Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S. Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes. Medicina Oral, Patologia Oral y Cirugia Bucal. 2017;22(2):e167–e176. [PMC free article: PMC5359698] [PubMed: 28160589]
- 4.
- Manger D, Walshaw M, Fitzgerald R, et al. Evidence summary: the relationship between oral health and pulmonary disease. British Dental Journal. 2017;222(7):527–533. [PubMed: 28387268]
- 5.
- Botero JE, Rodriguez C, Agudelo-Suarez AA. Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Australian Dental Journal. 2016;61(2):134–148. [PubMed: 26815303]
- 6.
- Faggion CM, Jr., Cullinan MP, Atieh M. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. Journal of Periodontal Research. 2016;51(6):716–725. [PubMed: 26913689]
- 7.
- Perez-Losada FL, Jane-Salas E, Sabater-Recolons MM, Estrugo-Devesa A, Segura-Egea JJ, Lopez-Lopez J. Correlation between periodontal disease management and metabolic control of type 2 diabetes mellitus. A systematic literature review. Medicina Oral, Patologia Oral y Cirugia Bucal. 2016;21(4):e440–446. [PMC free article: PMC4920457] [PubMed: 26827070]
- 8.
- Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016;17(1):31. [PMC free article: PMC4967318] [PubMed: 27473177]
- 9.
- Artese HP, Foz AM, Rabelo Mde S, et al. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PLoS ONE [Electronic Resource]. 2015;10(5):e0128344. [PMC free article: PMC4444100] [PubMed: 26010492]
- 10.
- Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials [Electronic Resource]. 2015;16:291. [PMC free article: PMC4490675] [PubMed: 26137892]
- 11.
- Mauri-Obradors E, Jane-Salas E, Sabater-Recolons Mdel M, Vinas M, Lopez-Lopez J. Effect of nonsurgical periodontal treatment on glycosylated hemoglobin in diabetic patients: a systematic review. Odontology/The Society of the Nippon Dental University. 2015;103(3):301–313. [PubMed: 25062756]
- 12.
- Schmitt A, Carra MC, Boutouyrie P, Bouchard P. Periodontitis and arterial stiffness: a systematic review and meta-analysis. Journal of Clinical Periodontology. 2015;42(11):977–987. [PubMed: 26465940]
- 13.
- Sun QY, Feng M, Zhang MZ, et al. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: a meta-analysis of randomized controlled trials. Chinese Journal of Physiology. 2014;57(6):305–314. [PubMed: 25575518]
- 14.
- Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine. 2014;93(28):e292. [PMC free article: PMC4603101] [PubMed: 25526470]
- 15.
- Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: A systematic review and meta-analysis. Journal of Diabetes Investigation. 2013;4(5):502–509. [PMC free article: PMC4025114] [PubMed: 24843701]
- 16.
- Liew AK, Punnanithinont N, Lee YC, Yang J. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Australian Dental Journal. 2013;58(3):350–357. [PubMed: 23981218]
- 17.
- Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):421–427. [PMC free article: PMC2809296] [PubMed: 20103557]
- 18.
- Ata-Ali F, Melo M, Cobo T, Nagasawa MA, Shibli JA, Ata-Ali J. Does Non-Surgical Periodontal Treatment Improve Glycemic Control? A Comprehensive Review of Meta-Analyses. Journal of the International Academy of Periodontology. 2020;22(4):205–222. [PubMed: 32980833]
- 19.
- Baeza M, Morales A, Cisterna C, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. Journal of Applied Oral Science. 2020;28:e20190248. [PMC free article: PMC6919200] [PubMed: 31939522]
- 20.
- Garde S, Akhter R, Nguyen MA, Chow CK, Eberhard J. Periodontal Therapy for Improving Lipid Profiles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019;20(15):05. [PMC free article: PMC6695858] [PubMed: 31387283]
- 21.
- Lima RPE, Belem FV, Abreu LG, et al. Effect of Periodontal Therapy on Serum Levels of IL-6 in Type 2 Diabetics: A Systematic Review. International Journal of Periodontics & Restorative Dentistry. 2019;39(1):e1–e10. [PubMed: 30543722]
- 22.
- Liu W, Cao Y, Dong L, et al. Periodontal therapy for primary or secondary prevention of cardiovascular disease in people with periodontitis. Cochrane Database of Systematic Reviews. 2019;12:CD009197. [PMC free article: PMC6953391] [PubMed: 31887786]
- 23.
- D’Aiuto F, Gable D, Syed Z, et al. Evidence summary: The relationship between oral diseases and diabetes. British Dental Journal. 2017;222(12):944–948. [PubMed: 28642531]
- 24.
- Simpson TC, Weldon JC, Worthington HV, et al. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database of Systematic Reviews. 2015(11):CD004714. [PMC free article: PMC6486035] [PubMed: 26545069]
- 25.
- D’Isidoro O, Perrotti V, Hui WL, Piattelli A, Iaculli F, Quaranta A. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: A literature review. American Journal of Dentistry. 2019;32(4):191–200. [PubMed: 31436940]
- 26.
- Agado BE, Crawford B, DeLaRosa J, et al. Effects of periodontal instrumentation on quality of life and illness in patients with chronic obstructive pulmonary disease: a pilot study. Journal of Dental Hygiene. 2012;86(3):204–214. [PubMed: 22947843]
- 27.
- Albert DA, Sadowsky D, Papapanou P, Conicella ML, Ward A. An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Services Research. 2006;6:103. [PMC free article: PMC1574303] [PubMed: 16914052]
- 28.
- Blaschke K, Hellmich M, Samel C, Listl S, Schubert I. The impact of periodontal treatment on healthcare costs in newly diagnosed diabetes patients: Evidence from a German claims database. Diabetes Research and Clinical Practice. 2021;172:108641. [PubMed: 33359573]
- 29.
- Choi H, Dey AK, Priyamvara A, et al. Role of Periodontal Infection, Inflammation and Immunity in Atherosclerosis. Current Problems in Cardiology. 2020:100638. [PMC free article: PMC8761259] [PubMed: 32646544]
- 30.
- Das AC, Das SJ, Panda S, Sharma D, Taschieri S, MD. F. Adjunctive Effect of Doxycycline with Conventional Periodontal Therapy on Glycemic Level for Chronic Periodontitis with Type 2 Diabetes Mellitus Subjects. Journal of Contemporary Dental Practice [Electronic Resource]. 2019;20(12):1417–1423. [PubMed: 32381843]
- 31.
- El-Makaky Y, Shalaby HK. The effects of non-surgical periodontal therapy on glycemic control in diabetic patients: A randomized controlled trial. Oral Diseases. 2020;26(4):822–829. [PubMed: 31834660]
- 32.
- Hsu YJ, Lin KD, Chen JH, et al. Periodontal Treatment Experience Associated with Oral Health-Related Quality of Life in Patients with Poor Glycemic Control in Type 2 Diabetes: A Case-Control Study. International Journal of Environmental Research & Public Health [Electronic Resource]. 2019;16(20):19. [PMC free article: PMC6843950] [PubMed: 31635118]
- 33.
- Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. American Journal of Preventive Medicine. 2014;47(2):166–174. [PubMed: 24953519]
- 34.
- Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. Journal of Periodontology. 2013;84(7):863–870. [PubMed: 23003917]
- 35.
- Lee YL, Hu HY, Huang N, Hwang DK, Chou P, Chu D. Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke. 2013;44(4):1026–1030. [PubMed: 23422085]
- 36.
- Lee JY, Choi YY, Choi Y, Jin BH. Efficacy of non-surgical treatment accompanied by professional toothbrushing in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Journal of Periodontal & Implant Science. 2020;50(2):83–96. [PMC free article: PMC7192821] [PubMed: 32395387]
- 37.
- Minassian C, D’Aiuto F, Hingorani AD, Smeeth L. Invasive dental treatment and risk for vascular events: a self-controlled case series. Annals of Internal Medicine. 2010;153(8):499–506. [PubMed: 20956706]
- 38.
- Mizuno H, Ekuni D, Maruyama T, et al. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial. Plos one. 2017;12(11). [PMC free article: PMC5689834] [PubMed: 29145468]
- 39.
- Nasseh K, Vujicic M, Glick M. The Relationship between Periodontal Interventions and Healthcare Costs and Utilization. Evidence from an Integrated Dental, Medical, and Pharmacy Commercial Claims Database. Health Economics. 2017;26(4):519–527. [PMC free article: PMC5347922] [PubMed: 26799518]
- 40.
- Peng CH, Yang YS, Chan KC, Kornelius E, Chiou JY, Huang CN. Periodontal Treatment and the Risks of Cardiovascular Disease in Patients with Type 2 Diabetes: A Retrospective Cohort Study. Internal Medicine. 2017;56(9):1015–1021. [PMC free article: PMC5478560] [PubMed: 28458305]
- 41.
- Smits KPJ, Listl S, Plachokova AS, Van der Galien O, Kalmus O. Effect of periodontal treatment on diabetes-related healthcare costs: a retrospective study. BMJ Open Diabetes Research & Care. 2020;8(1):10. [PMC free article: PMC7590362] [PubMed: 33099508]
- 42.
- Solowiej-Wedderburn J, Ide M, Pennington M. Cost-effectiveness of non-surgical periodontal therapy for patients with type 2 diabetes in the UK. Journal of Clinical Periodontology. 2017;44(7):700–707. [PubMed: 28504365]
- 43.
- Wang Y, Liu HN, Zhen Z, et al. A randomized controlled trial of the effects of non-surgical periodontal therapy on cardiac function assessed by echocardiography in type 2 diabetic patients. Journal of clinical periodontology. 2020. [PubMed: 32350903]
- 44.
- Healthcare U. Medical Dental Integration Study. https://www
.unitedhealthgroup .com/content /dam/UHG/PDF/2013/UHC-Medical-Dental-Integration-Study.pdf. Published 2013. Accessed 2021. - 45.
- Vergnes JN, Canceill T, Vinel A, et al. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. Journal of Clinical Periodontology. 2018;45(10):1150–1163. [PubMed: 30136741]
- 46.
- Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. Journal of Clinical Periodontology. 2014;41(6):564–572. [PubMed: 24593836]
Suggested citation:
Veazie S, Vela K, Parr NJ. Evidence Brief: Detection and Treatment of Dental Problems on Chronic Disease Outcomes. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-009; 2021. Available at: https://www.hsrd.research.va.gov/publications/esp/reports.cfm.
This report is based on research conducted by the Evidence Synthesis Program (ESP) Center located at the VA Portland Health Care System funded by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development. The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the United States government. Therefore, no statement in this article should be construed as an official position of the Department of Veterans Affairs. No investigators have any affiliations or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties) that conflict with material presented in the report.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Review Evidence Brief: Transcranial Magnetic Stimulation (TMS) for Chronic Pain, PTSD, TBI, Opioid Addiction, and Sexual Trauma[ 2020]Review Evidence Brief: Transcranial Magnetic Stimulation (TMS) for Chronic Pain, PTSD, TBI, Opioid Addiction, and Sexual TraumaAnderson J, Parr NJ, Vela K. 2020 Dec
- Review Evidence Brief: Doula Support for Veterans[ 2022]Review Evidence Brief: Doula Support for VeteransRahman B, Williams BE, Anderson JK, Ward RM, Mackey K, Parr NJ. 2022 Apr
- Review Evidence Brief: Virtual Diet Programs for Diabetes[ 2020]Review Evidence Brief: Virtual Diet Programs for DiabetesVeazie S, Vela K, Helfand M. 2020 Aug
- Review Evidence Brief: Traumatic Brain Injury and Dementia[ 2019]Review Evidence Brief: Traumatic Brain Injury and DementiaPeterson K, Veazie S, Bourne D, Anderson J. 2019 Feb
- Review Evidence Brief: Effectiveness of Stellate Ganglion Block for Treatment of Posttraumatic Stress Disorder (PTSD)[ 2017]Review Evidence Brief: Effectiveness of Stellate Ganglion Block for Treatment of Posttraumatic Stress Disorder (PTSD)Peterson K, Bourne D, Anderson J, Mackey K, Helfand M. 2017 Feb
- Evidence Brief: Detection and Treatment of Dental Problems on Chronic Disease Ou...Evidence Brief: Detection and Treatment of Dental Problems on Chronic Disease Outcomes
Your browsing activity is empty.
Activity recording is turned off.
See more...
