NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Microbial Threats. The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies. Washington (DC): National Academies Press (US); 2011.

The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies.
Show detailsIntroduction
The neglected tropical diseases (NTDs) are characterized by their prevalence among the poor of the most impoverished areas of the planet. These are neglected diseases of neglected populations, flourishing in the developing world but long since controlled or eradicated in the developed. NTDs generally affect the poorest people in poor societies—populations with little voice and representation. As a consequence, NTDs have until very recently received little funding and international attention. These diseases typically cause more disability than death. Their clinical features often lead to stigmatization and social isolation, which can further increase their neglect. Combined, as many as 1 billion people worldwide are at risk for NTDs or already suffer from them, and they account for 57 million disability-adjusted life-years lost (Hotez et al., 2007). However, these figures are thought to be an underestimate of the true burden (Hotez et al., 2008), because of a lack of reliable surveillance and of the research necessary to quantify their impacts (J. Jacobson, 2010).
As yet there is no internationally agreed consensus for which diseases come under this classification. In the first launch of a global report on NTDs, the World Health Organization (WHO) has defined 17 key diseases (WHO, 2010c), but several others exist. For the purposes of this review, we have opted to use the most inclusive definition possible, encompassing all NTDs that predominately affect the poor of the global south. In particular, we posit that malaria and poliomyelitis fit these criteria and may also be considered with the NTDs. Though both are less neglected than many other conditions, they affect many of the same populations as the classic NTDs, and they share many social and structural features with them.
Although evidence for the association between poverty and NTDs has been well documented, the impact of conflict on these diseases has been less well studied. With increasing prioritization of NTDs on the international agenda, these links are becoming more apparent. In the past few months, WHO has warned of several impending epidemics of NTDs in conflict settings. Of the four endemic countries for guinea worm, more than 90 percent of cases are currently in conflict-torn Sudan (CDC, 2010a). There has been a recent outbreak of visceral leishmainiasis in Southern Sudan (WHO, 2010b), a disease with high mortality among children. In a similar fashion, the number of cases of the cutaneous form of this disease has been soaring in Afghanistan as a result of the long-standing conflict there (WHO, 2010a).
Understanding the true burden of these diseases in conflict zones is difficult, as there are many political, logistical, and ethical barriers to conducting programs, surveillance, and research. Nonetheless, systematic evaluation of disease burden as well as effectiveness of interventions in the field is crucial to assist decisions with policy, advocacy, and providing care to these communities.
The human rights lens provides a useful ethical and legal framework to examine and address NTDs in conflict settings. Formal human rights conventions are a series of treaties, placing legal obligations on member states to ensure that they are accountable for their conduct. They define the humanitarian imperative for signatory states to respect, protect, and fulfill the basic human rights of their people. Most relevant among these instruments is the right to health, as defined by Article 12 of the 1976 International Covenant on Economic, Social and Cultural Rights. Specifically, Part c is applicable to NTDs, as it is defined by “[t]he prevention, treatment and control of epidemic, endemic, occupational and other diseases.” As of December 2010, 160 of the 192 United Nations member states were party to the Covenant. Like many processes mediated by the United Nations, enforcing these rights has proven to be difficult. Many countries worldwide are arguably in violation of the right to health. Nevertheless, the rights conventions do provide policy frameworks from which to advocate for more research into this area, legal tools for signatory state accountability, and platforms for action on global initiatives, such as the Millenium Development Goals.
We first explored this subject in 2007 (Beyrer et al., 2007). In this review we reassess the current literature and describe ways forward for responding to NTDs in conflict settings. In the first part, we analyze the literature in this field since 2007. In the second part we describe, through four case studies, potential mechanisms through which conflict can affect NTDs, and we review progress in the field.
Literature Review
Methods
We searched PubMed, without restriction on language for studies published between January 1, 2007, and October 15, 2010. Our search had two main components. To assess both the direct and indirect impacts of conflict, we used the following keywords: “conflict,” “war,” “civil unrest,” “political unrest,” “political instability,” “displacement camps,” “refugees,” “refugee camps,” and “internally displaced.” To assess articles that focused on NTDs, we used free text and MESH terms (where available), for all neglected diseases listed at http://www.plosntds.org.static/scope.action. We also included additional search terms on malaria and poliomyelitis.
As a further strategy we looked at recent morbidity and mortality surveys in known areas of conflict to see if NTDs or malaria were mentioned. We also cross-referenced with a recent WHO report (WHO, 2010c) and expert editorials (Hotez, 2009; Hotez and Kamath, 2009) to see if any references were missed.
Exclusion criteria were determined a priori: if the articles described pre-1980 conflicts, or described disease in non-endemic populations (i.e., military, tourists), they were excluded. We also chose not to include editorials, viewpoints, articles focusing on guidelines, isolated case reports, or letters. Also, if primary research was published in multiple journals, only one article was selected. Our full search protocol and results are summarized in Figure A2-1.
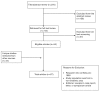
FIGURE A2-1
Search protocol and results.
Results
Table A2-1 describes new studies in the area of neglected diseases and conflict since 2007 (n = 67). Research was conducted in sub-Saharan Africa (51%), the Middle East (15%), North America and Australia (13%), Asia (11%), and on a global level (10%). The five most common disease areas investigated were malaria (39%), intestinal parasites (21%), leishmaniasis (12%), trachoma (7%), and trypanosomiasis (6%).
TABLE A2-1
Studies in Conflict and Neglected Tropical Diseases Since 2007.
Studies were either classified as primary research (70%), review articles (21%), and/or routine surveillance data (9%). The vast majority of primary research reports (45/47) were based on cross-sectional surveys to investigate the burden of disease in conflict settings.
The majority of papers identified an increased burden of disease in the conflict or postconflict environment. The only exception to this was a study by Mathenge et al. (2007) looking at causes of blindness in Rwanda, in which this association was not seen.
Mechanisms
On examination of the literature it is evident that conflict can impact on neglected diseases through several intermediate factors. These factors appear to act on the two principal points of the neglected disease cycle; either to increase exposure to the infectious agent and/or increase susceptibility to disease (see Box A2-1). As a result there is increased transmission of the neglected disease, and also more severe illness in individual patients.
BOX A2-1
The Impact of Conflict on Neglected Diseases. Factors affecting exposure to infectious agent Increased contact with vector (i.e., mosquito, tsetse fly)
The precise nature of the mechanisms may depend on the type of neglected disease involved. For example, the burden of vector-borne diseases (malaria, trypanosomiasis, and filariasis) may be increased in conflicts where there is substantial migration, and where there is a breakdown in control programs with limited access to prevention and treatment (Bygberg et al., 2010). Alternatively, the conditions of crowded refugee camps favor the transmission of water-borne diseases such as cholera and intestinal helminthes (Abu Mourad et al., 2008).
Furthermore it is important to note that the effects of conflict should be described in the wider context of other socioeconomic and ecological determinants of health in these settings. Poverty is a key factor along with conflict that impacts on the existing interplay between pathogen, human host, and the environment. In a recent study investigating the impact of the war in Côte d'Ivoire on risk factors for NTDs, Fürst has elegantly provided a conceptual framework to demonstrate these interactions (Figure A2-2).
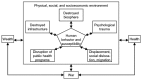
FIGURE A2-2
Conceptual framework for effect of conflict on NTDs. SOURCE: Reprinted from PLoS Neglected Tropical Diseases, Fürst et al. (2009).
Case studies can help to explore these mechanisms in further detail. Here we describe the impact conflict has had on the two common protozoan NTDs— trypanosomiasis and leishmaniasis—in diverse conflicts settings in Africa and Asia.
Case Example: Human African Trypanosomiasis
Human African trypanosomiasis (HAT) is endemic to 36 African countries, putting 60 million people at risk. If not diagnosed early, it causes a severe debilitating disease where mortality approaches 100 percent in untreated cases. It is estimated to cost Africa $1.5 billion in lost productivity. Treatment is available at low cost, and the disease can be successfully controlled with active population screening of cases and prompt administration of care (WHO, 2010).
In the 1960s, the disease was on the verge of eradication following effective disease control programs. However, lack of sustained disease control in the postindependence area, combined with periods of political unrest, led to resurgence of HAT cases in many countries. These cases peaked in 1996. Following several WHO resolutions in 1998 advocating for stronger access to diagnosis and treatment, the number of new cases reported had been reduced by 68 percent in 2005. Nonetheless, the disease continues to pose problems in conflict regions of Africa: In 2010, the three countries with the highest burden of HAT were Congo, Sudan, and Angola, all states with active conflicts or postconflict (WHO, 2010c).
Case Study: Sub-Saharan Africa
A recent study by Berrang-Ford and colleagues (2010) investigated the relationship between HAT incidence and conflict in Africa over a 30-year time period (1976–2004) using quantative epidemiological methods. In this study, the authors were able to obtain population-level data of both HAT incidence and conflicts within the region. In the study period, they demonstrated six significant space-time clusters of disease incidence (Figure A2-3). Four of the six clusters appear to be associated with specific conflicts, and it was postulated that one of the six clusters was indirectly associated (Table A2-2).

FIGURE A2-3
Map of the distribution of sleeping sickness incidence, Africa 1976–2004. SOURCE: Reprinted from Social Science & Medicine, Berrang-Ford et al., Conflict and human African trypanosomiasis, Copyright (2010), with permission from Elsevier. (more...)
TABLE A2-2
Summary of Six Space-Time Clusters of Sleeping Sickness Incidence, Africa 1976–2004.
The principal mechanism by which HAT incidence increases during and after a conflict appears because of a breakdown in disease control programs, such that transmission will continue because of inadequate diagnosis and treatment. However, as shown in the epidemic of disease in the Central African Republic, major population displacement due to conflicts in neighboring countries may also be an important factor.
The study by Berrang-Ford and colleagues is a major advancement in this field, because it uses quantative methods to assess the impact of conflict on the control of an NTD. Although epidemiological research of this nature cannot prove causality, and there may be several confounding factors to explain the findings (i.e., climatic factors, education, or political systems), it does provide convincing evidence to inform future policy and research.
Case Example: Cutaneous Leishmaniasis
Leishmaniasis is a disease caused by a group of intracellular parasites, which are transmitted from the bites of infected sandflies. Leishmania parasites can cause a spectrum of disease ranging from chronic ulcerating skin lesions (cutaneous leishmaniasis [CL]) or a disease that affects the internal organs of the body (visceral leishmaniasis [VL]), which is fatal if left untreated. There are an estimated 1.6 million cases annually (WHO, 2010c), and it is considered to be second in mortality and fourth in morbidity among all tropical diseases (Mathers et al., 2007).
Treatment of the disease is complex, involving drugs with a myriad of toxic side effects, although promising new regimens have recently been developed (WHO, 2010c). Control of the disease is possible through early access to diagnosis and treatment, as well as measures to reduce exposure to the vector, using periodic indoor spraying, health education, and bednets.
Conflict and civil unrest have been thought to be important factors driving leishmaniasis epidemics throughout the world (Bern et al., 2008). A resurgence in cases of VL has been documented in times of conflict in several sub-Saharan African counties, including northern Uganda, Somalia, and Chad (Hotez and Kamath, 2009). In the Middle East, VL has been documented to increase in wartorn areas in Palestine and Iraq, while CL is a major problem in Afghanistan, Iraq, and Pakistan (R. L.Jacobson, 2010).
Leishmaniasis thrives in conflict conditions, as a result of the breakdown in health infrastructure, forced migrations, destruction of human habitats, and food insecurity. Because of the collapse of health systems, patients are unable to access treatment, and there are few disease control measures available to reduce transmission. Poor housing, combined with a mobile population of refugees and internally displaced people, leads to greater transmission of the disease because of increased exposure to the sand-fly vector. In addition, there is a great deal of stress and malnutrition during war, which impairs the human body's defense system, increasing susceptibility to the disease.
Case Study: Afghanistan
Afghanistan has been engaged in conflict for more than 30 years. Health indicators are still among the worst globally, with many preventable diseases such as malaria, measles, and polio rampant throughout the country (Waldmann, 2002).
Kabul, the capital city, is currently the worldwide largest focus of CL with an estimated 67,500 new cases per annum (WHO, 2010a). The majority of CL in Afghanistan is anthroponotic, which means the human population is the main reservoir of infection. Because of the disfiguring nature of the skin disease it causes (i.e., large cutaneous lesions that appear at the biting site of the sand fly), the disease has a significant social impact on the local population. Individuals may become ostracized from society because of stigmatization, and thus the disease may effect their psychological, social, and economic well-being (Kassi et al., 2008). A questionnaire study of individuals in the capital city portrayed the many misconceptions that exist about how the disease is transmitted. Of the 360 respondents, the most common answers were touching (n = 86) and sharing meals (n = 26). As a result of these false beliefs, social exclusion is common. For example, women with lesions may be deemed unsuitable for marriage. They may be separated from their children during the disease, leading to depression and anxiety (Reithinger et al., 2005).
Several epidemics of CL have been described in Afghanistan, corresponding to increases in the intensity of conflict. An important factor in driving the high rates seen in Kabul has been forced migrations of susceptible individuals in the capital (Reyburn et al., 2003). In a similar fashion, outbreaks of disease have been described in neighboring Pakistan and Iran among Afghani refugees (Rowland et al., 1999).
Despite the fact that epidemics of CL have ravaged Afghanistan, there have been few disease control efforts. A few stories of successful public health interventions have been reported among the occupied military forces (Faulde et al., 2008). However, for the vast majority of Afghanis suffering from the disease in neglected areas of conflict, control programs have been non-existent. Because CL does not cause high rates of mortality, control of the disease has not been deemed cost-effective by WHO criteria, further increasing its neglect (Reithinger and Coleman, 2007).
However, control programs for leishmaniasis in conflict settings can be effective. In 2003, a major outbreak of VL was prevented in southern Iraq following the Allied invasion of the country (Jassim et al., 2006). As the burden of CL in Afghanistan expands and affects more vulnerable Afghans, it is imperative that we address the problem.
Ways Forward
In this final section, we highlight some potential interventions to improve the health of those suffering from NTDs in warzones.
Case Example: Intestinal Parasites
Intestinal parasites comprise both intestinal worms (nematodes) and protozoan infections. More than a quarter of the world's population is infected with nematodes (WHO, 2010c), and data on the true burden of protozoan infections are not known because many are not diagnosed. The vast majority of these diseases are transmitted via the feco-oral route, through contact with either contaminated water or food. Symptoms are often nonspecific in the early stages and only become evident when the infection is severe. Children and pregnant women are often the most susceptible. Effects include anemia, growth stunting, reduced physical fitness, impaired intellectual development, and poor educational performance (Feasey et al., 2010). Deworming treatment is available at low cost to treat these diseases, but access is very limited to people who need it the most. Preventative strategies focus on the provision of clean water, health education, and improved sanitary measures.
Conflict often results in large numbers of internally displaced individuals and refugees. These populations are ideal targets for intestinal parasites. Overcrowding, dirty water, minimal sanitary measures, and poor sewage systems are rife in displacement camps, creating the perfect environment for transmission. Mobile populations are often malnourished, increasing their susceptibility to more severe disease. Research in these settings has been limited; however, several cross-sectional surveys have shown a high burden of intestinal parasites in displacement camps in Sierra Leone (Gbakima et al., 2007), Sri Lanka (Chandrasena et al., 2007), and Palestine (Abu Mourad et al., 2008). In a similar fashion, high rates of these diseases are seen in refugees and asylum seekers when they reach developed countries including the United States, Canada, and Australia (Franco-Paredes et al., 2007; Posey et al., 2007; Pottie et al., 2007; Raman et al., 2009; Stauffer and Weinberg, 2009).
Case Study: Burma and Helminth Control in Internally Displaced People
The Burmese government has been engaged in civil war with ethnic minority groups for almost 50 years. There has been widespread documentation of human rights violations committed by the ruling government, including murder, torture, rape, forced labor, forced displacement, and destruction of villages (United Nations, 2010). As a result, a complex humanitarian crisis has developed, and there are estimated to be approximately 2 million internally displaced persons (IDPs). There is little sign of the crisis abating, with the recent reinstallment of the military government following what the international communities describe as “sham elections.”
As a result of this long-standing civil war, the health of these IDPs has suffered tremendously. The ruling party is estimated to allocate only 4 percent of the national budget on health care, even though it has been able to spend 40 percent on the military. Furthermore, in 2006 the government imposed new restrictions of international aid, forcing many aid agencies to leave the country (Stover et al., 2007). It is only since 2009 that humanitarian agencies have returned to be able to provide care. As a result, child and infant mortality rates in the ethnic zones are estimated to be some of the highest reported in the world (Lee et al., 2006). Compared to refugees, IDPs are a particularly difficult group for humanitarian agencies to access (Spiegel et al., 2010).
Infectious diseases are abundant, with malaria, HIV, filiariasis, and other neglected diseases being the principal health problems (Beyrer et al., 2006). Owing to the lack of permanent food, shelter, and clean water supply, mobile populations are particularly vulnerable to the effects of many of these diseases. Often it is pregnant women and children who suffer the most.
As a result of the high morbidity and mortality among IDPs living in Burma, local ethnic-based organizations have empowered themselves to provide health care for their respective communities. Through collaboration with international nongovernmental organizations (NGOs) and academic centers, they have set up control programs to tackle various health care problems in the ethnic zones of Burma. One such program is an innovative project to improve coverage of key maternal health services to improve reproductive health known as the the Mobile Obstetric Medics (MOM) project.
The MOM project was set up in 2005 following an initial survey exploring the very high maternal mortality rates seen in the ethnic zones of Burma. Intestinal parasitic infestation is thought to be an important cause of maternal anemia, leading to many pregnancy-related complications. At survey baseline, the presence of maternal anemia was shown to be 7 times more likely in those populations who had experienced food security violations than those who had not (Mullany et al., 2007).
Recently the first evaluation of the MOM project was described (Mullany et al., 2010). The researchers used a two-stage clustering survey to compare the delivery of key maternal interventions, before (2005) and after (2008) the program was implemented in four distinct ethnic areas in eastern Burma. One such intervention was deworming treatment for helminth infection: compared to baseline, pregnant women were 14.2 times (95% CI, 2.69–3.54) more likely to receive antihelminth coverage than before (Mullany et al., 2010). Since albendazole is a proven treatment for intestinal worms and there is no drug resistance, this is likely to have resulted in a reduction in helminth disease.
The MOM project provides an example of how community-based organizations have been effective in improving access to key interventions for helminth control for vulnerable populations in unstable conflict settings, which were previously considered inaccessible. This is particularly promising, as the MOM project may be used as a model in other settings to deliver simple interventions for neglected diseases.
Case Example: Malaria
Malaria is a protozoan disease that affects more than a third of the world's population and is estimated to cause approximately 1 million deaths annually—mainly among young children living in Africa (WHO, 2009). In resource-poor settings, the diagnosis traditionally has been made based on clinical symptoms alone, and confirmed by microscopy where available. In recent years, however, the introduction of rapid diagnostic tests has revolutionized diagnosis, allowing accurate diagnosis of malaria in remote field settings. Control programs are based on vector control measures such as insecticide-treated nets and health education, combined with early identification and treatment of cases. Although drug resistance to malaria has been a major problem, the use of artemisinin combination therapy (ACT) for the most severe form of malaria is still effective in most countries.
Malaria is fueled by conflict through several mechanisms. The breakdown in vector control programs and health infrastructure that occurs in wartime leads to increased transmission. In addition, the environmental destruction that occurs during a war is thought to encourage vector breeding (Rowland and Nosten, 2001). Forced displacement leads to exposure of nonimmune individuals to malaria-prevalent areas, leading to severe disease.
In 2000, WHO announced that approximately 30 percent of malaria deaths in Africa were a result of conflict or natural disasters. Malaria is often considered to be responsible for more deaths than the conflict itself. Outbreaks of malaria have recently been described in many conflict areas. Examples include the Democratic Republic of Congo (Coghlan et al., 2009), Afghanistan (Kolaczinski, 2005), and Burundi (Protopopoff et al., 2007). The increase in malaria incidence in refugees and displaced populations has been well described, for Iran (Basseri et al., 2010), Afghanistan (Basseri et al., 2010), Africa (Mouko et al., 2009), the United States (CDC, 2008), Israel (Kopel et al., 2010), and Jamaica (Lindo et al., 2007). Despite this fact, a recent study shows that almost 50 percent of national malaria strategic plans of African countries have no provision for refugees or internally displaced individuals (Spiegel et al., 2010).
The NGO sector has played a crucial role in delivering malaria care to these conflict areas where possible. There are even a few examples where NGOs have conducted basic clinical research to improve their control programs (Guthmann, 2009). However, it is not the primary agenda of NGOs to conduct such research. Furthermore, it may not be a sustainable solution, because NGOs are often only temporary providers.
The past few years have seen a dramatic increase in international funding for malaria. Despite this, in areas of conflict, malaria control remains underfunded and neglected.
Case Study: Timor Leste
During the civil conflict in Timor Leste in 2006, there was widespread street violence with more than 3,000 homes destroyed and displacement of approximately 15 percent of the country's population. In the capital city, Dili, more than 60 camps were established to provide temporary shelter for IDPs. These circumstances created the perfect environment for a malaria epidemic; however, through an array of control measures, such an epidemic was avoided (Martins et al., 2009).
Malaria in Timor Leste has always been a major public health problem. The disease incidence often follows a cyclical pattern with incidence increasing in the rainy season. In contrast to other conflict zones, the national malaria trends of 2006 showed no increase in malaria cases reported by the health system through the crisis (Figure A2-4).
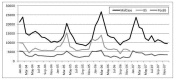
FIGURE A2-4
Malaria in Timor Leste, 2004–2007. SOURCE: Reprinted from Conflict and Health, Martins et al., Malaria control in Timor-Leste during a period of political instability: what lessons can be learned? 2009,3:11, published by BioMed Central Ltd.
The principal reason for this appears to be due to a well-galvanized and effective public health response, to prevent a malaria epidemic. This emergency response was co-coordinated centrally by the Ministry of Health (MOH) and involved all major development parties (MOH staff, WHO, international and national NGOs). Key to the success was the early provision of malaria interventions such as ACT treatment and massive ITN distribution to displacement camps. Although routine diseases surveillance was disrupted at the start of the crisis, the MOH ensured it was resumed as soon as possible, which was vital to ensure that the malaria epidemic could continue to be monitored.
Discussion
In this review, we have summarized interactions between NTDs and conflict. Conflict fuels NTDs for a variety of reasons, leading to increased acquisition of the infectious agent and increased susceptibility to disease.
Since our original assessment in 2007, there have been promising improvements in the quality of research in this area, enabling better characterization of the problem. Although many of the studies are cross-sectional, there has been an increase in the use of statistical modeling and geographic information systems, allowing diseases and conflicts to be mapped over space and time. New technology has enabled point-of-care testing for several NTDs, including malaria and trypanosomiasis. This has allowed novel methods for diagnosing these diseases in conflict settings but also better characterization of the total disease burden.
Almost all studies conducted in this field show an increase in NTDs in conflict and postconflict environments. Several studies have shown that, as a result of a conflict in one country, the control of the disease is often disrupted in the region as a whole. As such, it has been extremely difficult to enable worldwide eradication of NTDs such as guinea worm and polio, for these reasons.
There have also been some early examples of successful programmatic interventions for controlling NTDs in unstable settings. The MOM project in Burma provides an excellent model in which local communities have empowered themselves, using mobile health care workers, to provide antihelminth treatment to IDPs. With respect to vector-borne epidemics in conflict times, major outbreaks of malaria and leishmaniasis have been prevented by well-organized rapid-response measures. Ultimately, peace is the ideal intervention. However, initial outcomes may be deceptively poor as postconflict often means return of surveillance and a spike in “new” cases, which are really “newly detected cases.”
Despite improvements in research, this field remains underfunded and neglected. The human rights approach places a humanitarian imperative to address these issues. Working under the premise of “shared humanity,” it prioritizes the health and suffering of millions of IDPs, refugees, and victims of war. States can be made accountable to look after the health of all their individuals, regardless of their ethnicity or tribal affiliation.
Tackling NTDs in conflict should be part of a broader approach of improving basic rights to those living in conflict zones, who remain some of the most neglected individuals worldwide.
References
- Abu Mourad T, Radi S, Shashaa S, Lionis C, Philalithis A. Palestinian primary health care in light of the National Strategic Health Plan 1999–2003. Public Health. 2008;122:125–139. [PubMed: 17663010]
- Akello-Ayebare G, Richters JM, Polderman AM, Visser LG. Healthcare-seeking strategies among displaced children in war-ridden northern Uganda: The case of malaria. Annals of Tropical Medicine and Parasitology. 2010;104:369–376. [PubMed: 20819304]
- Al-Hucheimi SN, Sultan BA, Al-Dhalimi MA. A comparative study of the diagnosis of Old World cutaneous leishmaniasis in Iraq by polymerase chain reaction and microbiologic and histopathologic methods. International Journa of Dermatology. 2009;48:404–408. [PubMed: 19335428]
- Anwar M, Hussain MA, Ur-Rehman H, Khan I, Sheikh RA. Epidemic of cutaneous leishmaniasis: 109 cases in a population of 500. East Mediterranean Health Journal. 2007;13:1212–1215. [PubMed: 18290416]
- Basseri HR, Raeisi A, Holakouie K, Shanadeh K. Malaria prevention among Afghan refugees in a malarious area, southeastern Iran. Bulletin de la Societe de pathologie exotique. 2010;103(5):340–345. [PubMed: 20526828]
- Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Neglected Tropical Diseases. 2008;2:e313. [PMC free article: PMC2569207] [PubMed: 18958165]
- Berrang-Ford L. Civil conflict and sleeping sickness in Africa in general and Uganda in particular. Conflict Health. 2007;1:6. [PMC free article: PMC1851948] [PubMed: 17411421]
- Berrang-Ford L, Lundine J, Breau S. Conflict and human African trypanosomiasis. Social Science Medicine. 2010;73(3):398–407. [PubMed: 20619948]
- Beyrer C, Suwanvanichkij V, Mullany LC, Richards AK, Franck N, Samuels A, Lee TJ. Responding to AIDS, tuberculosis, malaria, and emerging infectious diseases in Burma: Dilemmas of policy and practice. PLoS Medicine. 2006;3:e393. [PMC free article: PMC1592343] [PubMed: 17032061]
- Beyrer C, Villar JC, Suwanvanichkij V, Singh S, Baral SD, Mills EJ. Neglected diseases, civil conflicts, and the right to health. Lancet. 2007;370:619–627. [PubMed: 17707757]
- Bompangue D, Giraudoux P, Piarroux M, Mutombo G, Shamavu R, Sudre B, et al. Cholera epidemics, war and disasters around Goma and Lake Kivu: An eight-year survey. PLoS Neglected Tropical Diseases. 2009;3:e436. [PMC free article: PMC2677153] [PubMed: 19436726]
- Brouqui P. Arthropod-borne disease associated with political and social disorder. Annual Review of Entomology. 2010;56:357–374. [PubMed: 20822446]
- Brun R, Blum J, Chappuis F, Burri C C. Human African trypanosomiasis. Lancet. 2010;375:148–159. [PubMed: 19833383]
- Bygbjerg IC. Conflicts and vector-borne diseases. Ugeskr Laeger. 2010;172:112–116. [PubMed: 20074487]
- CDC. Malaria in refugees from Tanzania—King County, Washington, 2007. MMWR. 2008;57:869–872. [PubMed: 18701876]
- CDC. Cholera outbreak—southern Sudan, 2007. MMWR. 2009;58:337–341. [PubMed: 19357634]
- CDC. Progress toward global eradication of dracunculiasis, January 2009–June 2010. MMWR. 2010;59:1239–1242. [PubMed: 20881936]
- CDC. Progress toward interruption of wild poliovirus transmission—worldwide, 2009. MMWR. 2010;59:545–550. [PubMed: 20467412]
- Chandrasena TG, Hapuarachchi HC, Dayanath MY, Pathmeswaran A, de Silva NR. Intestinal parasites and the growth status of internally displaced children in Sri Lanka. Tropical Doctor. 2007;37:163–165. [PubMed: 17716506]
- Clyti E, dos Santos RB. Endemic treponematoses in Maputo, Mozambique. Bulletin de la Societe de pathologie exotique. 2007;100:107–108. [PubMed: 17727031]
- Coghlan B, Ngoy P, Mulumba F, Hardy C, Bemo VN, Stewart T, et al. Update on mortality in the Democratic Republic of Congo: Results from a third nationwide survey. Disaster Medicine and Public Health Preparedness. 2009;3:88–96. [PubMed: 19491603]
- Colombatti R, Vieira CS, Bassani F, Cristofoli R, Coin A, Bertinato L, Riccardi F. Contamination of drinking water sources during the rainy season in an urban post-conflict community in Guinea Bissau: Implications for sanitation priority. African Journal of Medical Science. 2009;38:155–161. [PubMed: 20175419]
- Courtin F, Jamonneau V, Duvallet G, Camara M, Kaba D, Solano P. One century of “sleeping sickness” in West Africa. Bulletin de la Societe de pathologie exotique. 2008;101:287–289. [PubMed: 18681221]
- Denburg A, Rashid M, Brophy J, Curtis T, Malloy P, Audley J, et al. Initial health screening results for Karen refugees: A retrospective review. Canadian Communicable Disease Report. 2007;33:16–22. [PubMed: 18161207]
- Ekra KD, Attoh-Toure H, Benie BV, Coulibaly D, Koutouan MG, Aka LN, et al. Five years of cholera surveillance in Ivory Coast during social and political crisis, 2001 to 2005. Bulletin de la Societe de pathologie exotique. 2009;102:107–109. [PubMed: 19583033]
- Fair J, Jentes E, Inapogui A, Kourouma K, Goba A, Bah A, et al. Lassa virus-infected rodents in refugee camps in Guinea: A looming threat to public health in a politically unstable region. Vector Borne Zoonotic Diseases. 2007;7:167–171. [PubMed: 17627434]
- Faulde M, Schrader J, Heyl G, Amirih M. Differences in transmission seasons as an epidemiological tool for characterization of anthroponotic and zoonotic cutaneous leishmaniasis in northern Afghanistan. Acta Tropica. 2008;105:131–138. [PubMed: 18068684]
- Feasey N, Wansbrough-Jones M, Mabey DC, Solomon AW. Neglected tropical diseases. British Medical Bulletin. 2010;93:179–200. [PubMed: 20007668]
- Franco-Paredes C, Dismukes R, Nicolls D, Hidron A, Workowski K, Rodriguez-Morales A, et al. Persistent and untreated tropical infectious diseases among sudanese refugees in the United States. American Journal of Tropical Medicine and Hygiene. 2007;77:633–635. [PubMed: 17978062]
- Fürst T, Raso G, Acka CA, Tschannen AB, N'Goran EK, Utzinger J. Dynamics of eocioeconomic risk factors for neglected tropical diseases and malaria in an armed conflict. PLoS Neglected Tropical Diseases. 2009;3(9):e513. [PMC free article: PMC2731884] [PubMed: 19907632]
- Fürst T, Tschannen AB, Raso G, Acka CA, de Savigny D, Girardin O, et al. Effect of an armed conflict on relative socioeconomic position of rural households: Case study from western Cote d'Ivoire. Emerging Themes in Epidemiology. 2010;7:6. [PMC free article: PMC2945336] [PubMed: 20807398]
- Gayer M, Legros D, Formenty P, Connolly MA. Conflict and emerging infectious diseases. Emerging Infectious Diseases. 2007;13:1625–1631. [PMC free article: PMC3375795] [PubMed: 18217543]
- Gbakima AA, Konteh R, Kallon M, Mansaray H, Sahr F, Bah ZJ, et al. Intestinal protozoa and intestinal helminthic infections in displacement camps in Sierra Leone. African Journal of Medical Science. 2007;36:1–9. [PubMed: 17876913]
- Goswami ND, Shah JJ, Corey GR, Stout JE. Persistent eosinophilia and Strongyloides infection in Montagnard refugees after presumptive albendazole therapy. American Journal of Tropical Medicine and Hygiene. 2009;81:302–304. [PubMed: 19635888]
- Guthmann JP. Clinical research and humanitarian work: The role of Medecins sans Frontieres in the fight against malaria. Medical Science (Paris). 2009;25:301–306. [PubMed: 19361396]
- Hawkes M, Katsuva JP, Masumbuko CK. Use and limitations of malaria rapid diagnostic testing by community health workers in war-torn Democratic Republic of Congo. Malaria Journal. 2009;8:308. [PMC free article: PMC2804690] [PubMed: 20028563]
- Hotez PJ. The neglected tropical diseases and their devastating health and economic impact on the member nations of the Organisation of the Islamic Conference. PLoS Neglected Tropical Diseases. 2009;3:e539. [PMC free article: PMC2760759] [PubMed: 19859530]
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Neglected Tropical Diseases. 2009;3:e412. [PMC free article: PMC2727001] [PubMed: 19707588]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. New England Journal of Medicine. 2007;357:1018–1027. [PubMed: 17804846]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: The great neglected tropical diseases. Journal of Clinical Investigation. 2008;118:1311–1321. [PMC free article: PMC2276811] [PubMed: 18382743]
- Howard N, Shafi A, Jones C, Rowland M. Malaria control under the Taliban regime: Insecticide-treated net purchasing, coverage, and usage among men and women in eastern Afghanistan. Malaria Journal. 2010;9:7. [PMC free article: PMC2817706] [PubMed: 20053281]
- Jacobson J. Neglected and other infectious diseases. Paper presented at NTD Network Meeting; Atlanta, GA. September 23. 2010.
- Jacobson RL. Leishmaniasis in an era of conflict in the Middle East. Vector Borne Zoonotic Diseases. 2010 Epub. [PubMed: 20846030]
- Jassim AK, Maktoof R, Ali H, Budosan B, Campbell K. Visceral leishmaniasis control in Thi Qar Governorate, Iraq, 2003. East Mediterranean Health Journal. 2006;12(Suppl 2):S230–237. [PubMed: 17361695]
- Kakar F, Ahmadzai AH, Habib N, Taqdeer A, Hartman AF. A successful response to an outbreak of cholera in Afghanistan. Tropical Doctor. 2008;38:17–20. [PubMed: 18302855]
- Kassi M, Afghan AK, Rehman R, Kasi PM. Marring leishmaniasis: The stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Neglected Tropical Diseases. 2008;2:e259. [PMC free article: PMC2569210] [PubMed: 18958168]
- Khan SH, Goba A, Chu M, Roth C, Healing T, Marx A, et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Research. 2008;78:103–115. [PubMed: 18241935]
- Kibadi K, Panda M, Tamfum JJ, Fraga AG, Longatto Filho A, Anyo G, et al. New foci of Buruli ulcer, Angola and Democratic Republic of Congo. Emerging Infectious Diseases. 2008;14:1790–1792. [PMC free article: PMC2630729] [PubMed: 18976574]
- King JD, Ngondi J, Gatpan G, Lopidia B, Becknell S, Emerson PM. The burden of trachoma in Ayod County of Southern Sudan. PLoS Neglected Tropical Diseases. 2008;2:e299. [PMC free article: PMC2553487] [PubMed: 18820746]
- Kolaczinski J. Roll back Malaria in the aftermath of complex emergencies: The example of Afghanistan. Tropical Medicine International Health. 2005;10:888–893. [PubMed: 16135196]
- Kolaczinski JH, Reithinger R, Worku DT, Ocheng A, Kasimiro J, Kabatereine N, Brooker S. Risk factors of visceral leishmaniasis in East Africa: A case-control study in Pokot territory of Kenya and Uganda. International Journal of Epidemiology. 2008;37:344–352. [PMC free article: PMC2637948] [PubMed: 18184669]
- Kopel E, Schwartz E, Amitai Z, Volovik I. Relapsing vivax malaria cluster in Eritrean refugees, Israel, June 2010. Eurosurveillance. 2010;15 [PubMed: 20619133]
- Kruk ME, Rockers PC, Williams EH, Varpilah ST, Macauley R, Saydee G, Galea S. Availability of essential health services in post-conflict Liberia. Bulletin of the World Health Organization. 2010;88:527–534. [PMC free article: PMC2897988] [PubMed: 20616972]
- Kur LW, Picon D, Adibo O, Robinson E, Sabasio A, Edwards T, et al. Trachoma in Western Equatoria State, Southern Sudan: Implications for national control. PLoS Neglected Tropical Diseases. 2009;3:e492. [PMC free article: PMC2710503] [PubMed: 19636366]
- Lee CI, Smith LS, Shwe Oo EK, Scharschmidt BC, Whichard E, Kler T, et al. Internally displaced human resources for health: Villager health worker partnerships to scale up a malaria control programme in active conflict areas of eastern Burma. Global Public Health. 2009;4:229–241. [PubMed: 19384681]
- Lee TJ, Mullany LC, Richards AK, Kuiper HK, Maung C, Beyrer C. Mortality rates in conflict zones in Karen, Karenni, and Mon states in eastern Burma. Tropical Medicine International Health. 2006;11:1119–1127. [PubMed: 16827712]
- Leslie T, Kaur H, Mohammed N, Kolaczinski K, Ord RL, Rowland M. Epidemic of Plasmodium falciparum malaria involving substandard antimalarial drugs, Pakistan, 2003. Emerging Infectious Diseases. 2009;15:1753–1759. [PMC free article: PMC2857251] [PubMed: 19891862]
- Lindo JF, Bryce JH, Ducasse MB, Howitt C, Barrett DM, Lorenzo Morales J, et al. Plasmodium malariae in Haitian refugees, Jamaica. Emerging Infectious Diseases. 2007;13:931–933. [PMC free article: PMC2792841] [PubMed: 17553241]
- Martins JS, Zwi AB, Martins N, Kelly PM. Malaria control in Timor-Leste during a period of political instability: What lessons can be learned? Conflict Health. 2009;3:11. [PMC free article: PMC2802356] [PubMed: 20003539]
- Mathenge W, Nkurikiye J, Limburg H, Kuper H. Rapid assessment of avoidable blindness in Western Rwanda: Blindness in a postconflict setting. PLoS Medicine. 2007;4:e217. [PMC free article: PMC1904464] [PubMed: 17608561]
- Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: The global burden of disease framework. PLoS Neglected Tropical Diseases. 2007;1:e114. [PMC free article: PMC2100367] [PubMed: 18060077]
- Mouko A, Mbika-Cardorelle A, Mboungou V, Mambou JB, Ibara JR, Senga P. Orphans in Brazzaville orphanages. Sante. 2009;19:21–23. [PubMed: 19801347]
- Mullany LC, Richards AK, Lee CI, Suwanvanichkij V, Maung C, Mahn M, et al. Population-based survey methods to quantify associations between human rights violations and health outcomes among internally displaced persons in eastern Burma. Journal of Epidemiology Community Health. 2007;61:908–914. [PMC free article: PMC2652972] [PubMed: 17873229]
- Mullany LC, Lee TJ, Yone L, Lee CI, Teela KC, Paw P, et al. Impact of community-based maternal health workers on coverage of essential maternal health interventions among internally displaced communities in Eastern Burma: The MOM project. PLoS Medicine. 2010;7:e1000317. [PMC free article: PMC2914639] [PubMed: 20689805]
- Mumtaz K, Kamani L, Chawla T, Hamid S, Jafri W. Hepatic cystic echinococcosis: Clinical characteristics and outcomes in Pakistan. Tropical Doctor. 2009;39:215–217. [PubMed: 19762573]
- Ngondi J, Matthews F, Reacher M, Onsarigo A, Matende I, Baba S, et al. Prevalence of risk factors and severity of active trachoma in southern Sudan: An ordinal analysis. American Journal of Tropical Medicine and Hygiene. 2007;77:126–132. [PubMed: 17620643]
- Ngondi JM, Matthews FE, Reacher MH, King J, Brayne C, Gouda H, Emerson PM. What will happen if we do nothing to control trachoma: Health expectancies for blinding trachoma in southern Sudan. PLoS Neglected Tropical Diseases Dis. 2009;3:e396. [PMC free article: PMC2652411] [PubMed: 19290039]
- Posey DL, Blackburn BG, Weinberg M, Flagg EW, Ortega L, Wilson M, et al. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clinical Infectious Diseases. 2007;45:1310–1315. [PubMed: 17968826]
- Pottie K, Janakiram P, Topp P, McCarthy A. Prevalence of selected preventable and treatable diseases among government-assisted refugees: Implications for primary care providers. Canadian Family Physician. 2007;53:1928–1934. [PMC free article: PMC2231488] [PubMed: 18000270]
- Protopopoff N, Van Herp M, Maes P, Reid T, Baza D, D'Alessandro U, et al. Vector control in a malaria epidemic occurring within a complex emergency situation in Burundi: A case study. Malaria Journal. 2007;6:93. [PMC free article: PMC1971709] [PubMed: 17634116]
- Raman S, Wood N, Webber M, Taylor KA, Isaacs D. Matching health needs of refugee children with services: How big is the gap? Australia New Zealand Journal of Public Health. 2009;33:466–470. [PubMed: 19811485]
- Reithinger R, Coleman PG. Treating cutaneous leishmaniasis patients in Kabul, Afghanistan: Cost-effectiveness of an operational program in a complex emergency setting. BMC Infect Dis. 2007;7:3. [PMC free article: PMC1790896] [PubMed: 17263879]
- Reithinger R, Aadil K, Kolaczinski J, Mohsen M, Hami S. Social impact of leishmaniasis, Afghanistan. Emerging Infectious Diseases. 2005;11:634–636. [PMC free article: PMC3320322] [PubMed: 15834984]
- Reithinger R, Mohsen M, Leslie T. Risk factors for anthroponotic cutaneous Leishmaniasis at the household level in Kabul, Afghanistan. PLoS Neglected Tropical Diseases. 2010;4:e639. [PMC free article: PMC2843638] [PubMed: 20351787]
- Reyburn H, Rowland M, Mohsen M, Khan B, Davies C. The prolonged epidemic of anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: “Bringing down the neighbourhood” Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:170–176. [PubMed: 14584372]
- Richards AK, Smith L, Mullany LC, Lee CI, Whichard E, Banek K, et al. Prevalence of Plasmodium falciparum in active conflict areas of eastern Burma: A summary of cross-sectional data. Conflict Health. 2007;1:9. [PMC free article: PMC2034373] [PubMed: 17803819]
- Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Annals Tropical Medicine Parasitology. 2001;95:741–754. [PubMed: 11784429]
- Rowland M, Munir A, Durrani N, Noyes H, Reyburn H. An outbreak of cutaneous leishmaniasis in an Afghan refugee settlement in north-west Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:133–136. [PubMed: 10450434]
- Rumunu J, Brooker S, Hopkins A, Chane F, Emerson P, Kolaczinski J. Southern Sudan: An opportunity for NTD control and elimination? Trends in Parasitology. 2009;25:301–307. [PMC free article: PMC2729080] [PubMed: 19540164]
- Shikanga OT, Mutonga D, Abade M, Amwayi S, Ope M, Limo H, et al. High mortality in a cholera outbreak in western Kenya after post-election violence in 2008. American Journal of Tropical Medicine and Hygiene. 2009;81:1085–1090. [PubMed: 19996441]
- Shultz A, Omollo JO, Burke H, Qassim M, Ochieng JB, Weinberg M, et al. Cholera outbreak in Kenyan refugee camp: Risk factors for illness and importance of sanitation. American Journal of Tropical Medicine and Hygiene. 2009;80:640–645. [PubMed: 19346392]
- Spiegel PB, Hering H, Paik E, Schilperoord M. Conflict-affected displaced persons need to benefit more from HIV and malaria national strategic plans and Global Fund grants. Conflict Health. 2010;4:2. [PMC free article: PMC2827465] [PubMed: 20205901]
- Stauffer WM, Weinberg M. Emerging clinical issues in refugees. Current Opinions in Infectious Diseases. 2009;22:436–442. [PubMed: 19587590]
- Stover E, Suwanvanichkij V, Moss A. The Gathering Storm: Infectious Diseases and Human Rights in Burma. Human Rights Center University of California Berkeley, and Center for Public Health and Human Rights, Johns Hopkins Bloomberg School of Public Health; 2007.
- Sturrock HJ, Picon D, Sabasio A, Oguttu D, Robinson E, Lado M, et al. Integrated mapping of neglected tropical diseases: Epidemiological findings and control implications for northern Bahr-el-Ghazal State, Southern Sudan. PLoS Neglected Tropical Diseases. 2009;3:e537. [PMC free article: PMC2761732] [PubMed: 19859537]
- Suykerbuyk P, Wambacq J, Phanzu DM, Haruna H, Nakazawa Y, Ooms K, et al. Persistence of Mycobacterium ulcerans disease (Buruli ulcer) in the historical focus of Kasongo Territory, the Democratic Republic of Congo. American Journal of Tropical Medicine and Hygiene. 2009;81:888–894. [PubMed: 19861627]
- United Nations. The Special Rapporteur of the Commission on Human Rights on the situation of human rights in Myanmar. 2010 UN Doc A/65/368 (15 Sep 2010)
- Varkey P, Jerath AU, Bagniewski S, Lesnick T. Intestinal parasitic infection among new refugees to Minnesota, 1996–2001. Travel Medicine and Infectious Disease. 2007;5:223–229. [PubMed: 17574143]
- Waldmann RHH. The Public Health System in Afghanistan—Current Issues. Afghanistan Research Evaluation Unit; Islamabad: 2002. pp. 1–46. (Issues Paper).
- WHO (World Health Organization). World Malaria Report. WHO; Geneva: 2009.
- WHO (World Health Organization). 13 million Afghans at risk of contracting leishmaniasis, says WHO. WHO press release. 2010.
- WHO (World Health Organization). Upsurge of kala-azar cases in Southern Sudan requires rapid response. World Health Organisation press report. 2010 October 8
- WHO (World Health Organization). Working to overcome the global impact of neglected tropical diseases, First WHO Report on Neglected Tropical Diseases. WHO; Geneva: 2010.
- 1
Corresponding Author: Chris Beyrer MD, MPH, Dept. of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD 21205, USA.
- NEGLECTED TROPICAL DISEASES, CONFLICT, AND THE RIGHT TO HEALTH - The Causes and ...NEGLECTED TROPICAL DISEASES, CONFLICT, AND THE RIGHT TO HEALTH - The Causes and Impacts of Neglected Tropical and Zoonotic Diseases
Your browsing activity is empty.
Activity recording is turned off.
See more...