NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lamprecht M, editor. Antioxidants in Sport Nutrition. Boca Raton (FL): CRC Press/Taylor & Francis; 2015.
7.1. INTRODUCTION
Free radicals are commonly thought of as perpetrators of cell damage, ageing and even cancer, while antioxidants are seen as the defence against these threats. As awareness about the harmful effects of free radicals has increased, so has consciousness regarding the importance of dietary antioxidants. As a result, many health-conscious people turn to nutritional supplements containing vitamins and other antioxidants. Particular interest in antioxidant supplements has arisen among athletes and people who train regularly. Indeed, antioxidants are among the most common sport supplements used by amateur and professional athletes (Krumbach et al. 1999; Margaritis and Rousseau 2008; Sobal and Marquart 1994). A quick survey of the labels on most energy bars and recovery drinks would seem to erase any doubt that athletes have an extraordinary need for antioxidants. However, it has not been conclusively shown that this is the case, and it remains debatable whether large amounts of supplementary antioxidants are sensible at all for athletes in training (Padilla and Mickleborough 2007).
Indeed, there is a growing body of evidence that the appearance of free radicals in skeletal muscle, besides having certain negative effects, also fulfils important physiological functions in cells, and that the right balance between antioxidants and free radicals is necessary for the desired physiological adaptations (Gomez-Cabrera et al. 2008; Ji 2008). Thus, it becomes necessary to assess the prudence of antioxidant supplementation, particularly among athletes in training (Gross et al. 2011).
In this chapter, we briefly address the production of free radicals at rest as well as during and in response to exercise or training. Then we reflect upon the negative and positive effects free radicals can have on skeletal muscles, highlighting the signalling functions of free radicals in the process of physiological adaptation to training. Finally, the influence of supplemental antioxidants on free radical biology and training adaptations is discussed. An overview of the situation discussed in this chapter is presented in Figure 7.1.

FIGURE 7.1
Overview of the interaction between free radicals (ROS/RNS), antioxidants and their effects.
7.2. FREE RADICAL PRODUCTION IN SKELETAL MUSCLE
Free radicals are a heterogeneous group of molecules that are characterised by a free valence electron in their outer atom orbital. Owing to this unpaired electron, free radicals may rapidly react with other molecules. Accordingly, their biological half-life time is very short in biological solutions, including cellular surroundings.
In skeletal muscle fibres, three main free radicals are found: nitric oxide (NO•) as the main representative of reactive nitrogen species (RNS) as well as superoxide anion  and hydroxyl radicals (•OH) as the most important reactive oxygen species (ROS). Hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) also belong to the group of ROS without being free radicals.
and hydroxyl radicals (•OH) as the most important reactive oxygen species (ROS). Hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) also belong to the group of ROS without being free radicals.
The gaseous NO• is generated by NADPH-dependent oxidation of l-arginine by the catalytic activity of intracellular NO synthases (NOS). Skeletal muscle contains high concentrations of neuronal NOS (nNOS) anchored at the sarcolemma. Additionally, NO• may be generated in skeletal muscle by endothelial NOS (eNOS), which is expressed in endothelial cells of arterial and venous vascular sections and capillaries.
 represents the top of the ROS cascade, which is mainly generated by the activity of membrane-associated NADPH oxidase and as a by-product by the respiratory chain complexes I and III (for review, see Jackson et al. 2007; Powers et al. 2011) in the mitochondria. Superoxide is highly toxic, as it inactivates enzymes that contain iron–sulphur clusters as a prosthetic group (Valko et al. 2007). Therefore, the rapid detoxification of superoxide is of physiological relevance. The reaction of
represents the top of the ROS cascade, which is mainly generated by the activity of membrane-associated NADPH oxidase and as a by-product by the respiratory chain complexes I and III (for review, see Jackson et al. 2007; Powers et al. 2011) in the mitochondria. Superoxide is highly toxic, as it inactivates enzymes that contain iron–sulphur clusters as a prosthetic group (Valko et al. 2007). Therefore, the rapid detoxification of superoxide is of physiological relevance. The reaction of  with NO, which yields ONOO−, represents one possible mode of elimination. This reaction is approximately 3 times more efficient at scavenging superoxide than is that catalysed by the superoxide dismutase (SOD) system (Pattwell et al. 2004).
with NO, which yields ONOO−, represents one possible mode of elimination. This reaction is approximately 3 times more efficient at scavenging superoxide than is that catalysed by the superoxide dismutase (SOD) system (Pattwell et al. 2004).
Various enzymes generate significant levels of H2O2 expressed in skeletal muscle fibres (for review, see Jackson et al. 2007). The most important are the three forms of SOD: the sarcoplasm SOD-1 and the mitochondrial SOD-2 require copper and zinc on their active side, while the manganese-dependent SOD-3 is located in the extracellular fluid.
Far less is known about the biology of •OH. Presumably, in a microsomal compartment in the presence of Fe2+, this ROS is generated non-enzymatically from H2O2 and very rapidly scavenged by endogenous antioxidants.
Studies using nNOS-knockout mice and nNOS-overexpressing transgenic mice have previously shown that an alteration of the nNOS expression levels in skeletal muscle is accompanied by a change in the availability of  and, subsequently, H2O2 (SOD-1 dependent) and/or ONOO− (Da Silva-Azevedo et al. 2009; Sakellariou et al. 2011). These studies suggest that levels of NO• and ROS may influence each other, indicating that free radical metabolism is highly regulated and balanced in muscle tissue.
and, subsequently, H2O2 (SOD-1 dependent) and/or ONOO− (Da Silva-Azevedo et al. 2009; Sakellariou et al. 2011). These studies suggest that levels of NO• and ROS may influence each other, indicating that free radical metabolism is highly regulated and balanced in muscle tissue.
7.3. NEGATIVE EFFECTS OF FREE RADICALS
High concentrations of free radicals, in particular those of rapidly released ROS, lead to a strong oxidative stress in cells, for example, in neutrophils and monocytes of the immune system but also in skeletal muscle. This is useful for defending against microbiological pathogens, and while the abrupt increase in oxidants helps eliminate infiltrating cells, it also harms the defending cells, which are not protected against this burst of oxidants. Even low levels of ROS might induce cell damage by chemical inactivation of important molecules such as deoxyribonucleic acid (DNA) by base damage and single-strand breaks, unsaturated fatty acids by lipid peroxidation or amino acids and cofactors in proteins by oxidation.
Free radicals may also bring the cellular homeostasis out of balance indirectly. The formation of ONOO− reduces the levels of bioactive NO•, which is required to dilate terminal arterioles, feed arteries and resistance arteries. In addition,  and ONOO− also lead to apoptosis or inflammatory responses via activation of redox-sensitive signalling cascades. Accordingly, imbalance of the ROS metabolism is associated with cardiovascular diseases, stroke and heart attack.
and ONOO− also lead to apoptosis or inflammatory responses via activation of redox-sensitive signalling cascades. Accordingly, imbalance of the ROS metabolism is associated with cardiovascular diseases, stroke and heart attack.
7.4. SIGNALLING FUNCTIONS OF FREE RADICALS
Although free radicals have traditionally been considered mainly as a threat to cell stability and health, results from several recent studies present the framework for a functional role of free radicals, such as NO• and  as well as H2O2, as important cell-signalling molecules. Endogenous oxidant defence is up-regulated by negative feedback from ROS, especially
as well as H2O2, as important cell-signalling molecules. Endogenous oxidant defence is up-regulated by negative feedback from ROS, especially  (Gomez-Cabrera et al. 2008), but free radicals also play an important role in stimulating physiological adaptation, especially those related to endurance training. With endurance exercise, increased flux of oxygen through mitochondria causes acute increases in
(Gomez-Cabrera et al. 2008), but free radicals also play an important role in stimulating physiological adaptation, especially those related to endurance training. With endurance exercise, increased flux of oxygen through mitochondria causes acute increases in  production; exercise-associated depletion of substrates, leading to a drop in glutathione reductase activity, and hyperthermia, which promotes mitochondrial uncoupling and loss of respiratory control, can also contribute to additional free radical production during exercise. Further, transient hypoxia during anaerobic exercise leading to acidosis, as well as reperfusion of hypoxic muscle, can increase oxidative stress (Kanter 1998). Finally, the mechanical stress of exercise can itself increase free radical formation (Symons 1988). These are all phenomena which occur due to training and help provide the stimulus for physiological adaptation.
production; exercise-associated depletion of substrates, leading to a drop in glutathione reductase activity, and hyperthermia, which promotes mitochondrial uncoupling and loss of respiratory control, can also contribute to additional free radical production during exercise. Further, transient hypoxia during anaerobic exercise leading to acidosis, as well as reperfusion of hypoxic muscle, can increase oxidative stress (Kanter 1998). Finally, the mechanical stress of exercise can itself increase free radical formation (Symons 1988). These are all phenomena which occur due to training and help provide the stimulus for physiological adaptation.
Further, free radicals may act in the extracellular space; for example, NO• induces vasodilation in feeding and resistance arteries (Clifford and Hellsten 2004), leading to increased blood-flow velocity (Kayar et al. 1992). The resulting increase in shear stress in the microvasculature of muscle fibres is an important stimulus for angiogenesis in muscle (Baum et al. 2004). ROS also affects the metabolism of carbohydrates and lipids by regulation of the mRNA expression levels of key enzymes (Hoppeler et al. 2011), and there is increasing evidence suggesting that they play an important role in modulating redox-sensitive signalling pathways on the way to further muscular adaptations (Jackson 2009). Also, ROS helps to stimulate the up-regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha), a master regulatory gene of mitochondrial and vascular adaptation, via the 5′ adenosine monophosphate-activated protein kinase (AMPK) pathway (Hoppeler et al. 2011).
The most intensively characterised signal transduction pathway triggered by NO• comprises the activation of soluble guanyl cyclase which, via its synthesis product cyclic guanosine monophosphate (cGMP), subsequently increases the activity of the cGMP-dependent protein kinase G (PKG). However, NO• might also covalently bind to amino acids within the primary structure of other signalling enzymes. These post-translational modifications, for example, cysteine S-nitrosylation (reversible) and tyrosine nitration (irreversible), enable the initiation of subsequent triggering of cellular cascades (Godecke et al. 2008; Stamler et al. 2008). In the skeletal muscle of humans, NO• production has been identified to directly regulate the transcription rate of stress genes after exercise (Steensberg et al. 2007).
Adaptations of the free radical metabolism following training may be dependent on changes to cellular thiol:disulphide ratios, or redox potentials, caused by free radicals (Jackson 2009) or the transient appearance of  (Gomez-Cabrera et al. 2008), as these appear to stimulate the up-regulation of certain important transcription factors within this pathway. ROS levels after aerobic endurance training have been associated with muscle and heart hypertrophy, angiogenesis and glucose transport ability (Gibala 2009; Ji 2007). Furthermore, they seem to provide signals for the expression of genes related to metabolism, as will be addressed below.
(Gomez-Cabrera et al. 2008), as these appear to stimulate the up-regulation of certain important transcription factors within this pathway. ROS levels after aerobic endurance training have been associated with muscle and heart hypertrophy, angiogenesis and glucose transport ability (Gibala 2009; Ji 2007). Furthermore, they seem to provide signals for the expression of genes related to metabolism, as will be addressed below.
Free radicals may also have acute positive effects on contractility and improvement of performance. In low concentrations, they help maintain muscle force production (Jackson 2009; Powers and Jackson 2008). Further, during the oxidative burst of phagocytosis, macrophages release  , H2O2 and NO• as part of the clearing out of damaged or dead cell material, which helps to speed up the repair process (Valko et al. 2006).
, H2O2 and NO• as part of the clearing out of damaged or dead cell material, which helps to speed up the repair process (Valko et al. 2006).
7.5. EFFECTS OF SUPPLEMENTAL ANTIOXIDANTS ON TRAINING ADAPTATIONS
The negative effects discussed above, which can be exhibited by RNS and ROS, supposedly provide the basis for the beneficial effect of supplemental antioxidants such as vitamins C and E or β-carotene (non-enzymatic antioxidants, which are not synthesised in humans and must be obtained exogenously). These substances are able to scavenge various free radicals by proton donation (Sies and Stahl 1995).
There have been some benefits of vitamin supplements in certain populations or situations. It has been shown that vitamin C can help strengthen immune defence (Kreider et al. 2004; Valko et al. 2006) while vitamin E could enhance energy balance at high altitude (Simon-Schnass and Pabst 1988). Further, the two function together to protect against lipid peroxidation. Vitamin A is particularly well suited for scavenging  , •OH and peroxyl radicals such as ONOO− (Valko et al. 2004).
, •OH and peroxyl radicals such as ONOO− (Valko et al. 2004).
However, evidence from clinical studies does not support a protective effect of vitamins C and E or β-carotene against DNA damage or cancer (Valko et al. 2004) or against cardiovascular disease (Myung et al. 2013). Further, it should be noted that under certain circumstances, these antioxidants may become pro-oxidative agents. β-carotene, in the presence of increased partial pressure of oxygen, can be converted into a peroxyl radical, and vitamin C can form DNA-damaging genotoxins from lipid hydroperoxides in the presence of transition metal ions (Valko et al. 2004).
Further, questions have been raised about the efficacy of high doses of supplemental dietary antioxidants such as vitamins C and E during endurance training. In some cases, counteracting ROS accumulation via acute antioxidant supplementation can positively affect athletic performance. For example, pharmacologically boosting the capacity to convert H2O2 into water protects against ROS-induced fatigue or enhances time to exhaustion (Medved et al. 2004; Reid 2008). However, most such studies employ intravenous infusions instead of common oral supplements. In general, evidence does not support the belief that vitamins and antioxidants are ergogenic or contribute to enhanced training effectiveness. The consensus is that supplemental vitamins C and E and ubiquinone are not ergogenic in normally nourished athletes at low altitude and vitamins C and E and β-carotene do not prevent training-induced muscle damage in humans (Williams 2004).
Moreover, several publications suggest that these may actually be counterproductive (Gomez-Cabrera et al. 2005, 2008; Ji 2008; Ristow et al. 2009; Wray et al. 2009), since it is supposed that radicals such as ROS and NO• play an important signalling role for metabolism, mitochondriogenesis and angiogenesis, and artificial suppression with supplemental antioxidants may weaken these signals. For example, one response to the elevated oxidative stress associated with exercise is increased oxidant defence via up-regulation of antioxidant enzymes such as SOD and glutathione peroxidase (GPx). However, antioxidant supplementation discourages such adaptations by interfering with the radical-mediated signal (Gomez-Cabrera et al. 2005, 2008). The importance of this consequence may not be obvious if one assumes a surrogate protective effect from exogenous antioxidants; however, endogenous mechanisms could be more important when radical production is particularly high. Accordingly, significantly greater oxidative damage has been observed following half and full ironman triathlons in athletes who took antioxidant supplements than in those who did not (2007).
The challenges faced by energy production systems during training stimulate enhanced capacity through the likes of increased mitochondrial volume density and capillarisation of muscle fibres, and improved provision and utilisation of the substrate. Here too, placebo-controlled studies with animals and humans provide evidence for interference of orally supplemented antioxidants, including vitamin C on exercise-induced signalling and dependent events such as expression of the mitochondrial enzyme cytochrome C, which is representative of mitochondrial volume (Gomez-Cabrera et al. 2008), and improvements to insulin sensitivity (Ristow et al. 2009). Elsewhere, in humans involved in an endurance-training programme, acute supplementation of vitamins C and E seemed to prevent exercise-induced vasodilation (Wray et al. 2009), which, in addition to causing acute hypertension, could also blunt the blood-flow-induced stimulus for angiogenesis. Moreover, angiogenesis is prevented if NO• release from eNOS is blocked (Baum et al. 2004; Hudlicka et al. 2000).
As discussed above, PGC-1 alpha is an established inducer of mitochondriogenesis (Handschin and Spiegelman 2008) and the expression of the gene for PGC-1α increases with training. However, this response is suppressed or eliminated by the administration of supplemental antioxidants in mice (Meier et al. 2013), rats (Gomez-Cabrera et al. 2008) and humans (Ristow et al. 2009), which suggests that PGC-1 alpha mRNA expression is favoured by ROS and that the up-regulation of PGC-1 alpha, in particular, in response to endurance exercise is very sensitive to the redox state of the skeletal muscle. This being the case, mitochondrial proteins may nonetheless increase with training combined with antioxidant supplementation, despite interference of antioxidants with PGC-1a mRNA expression, as has been observed in humans and rats (Gomez-Cabrera et al. 2008; Irrcher et al. 2009; Powers et al. 2011). Possibly, sustained activation of PGC-1 alpha at the post-translational level (phosphorylation, acetylation) (Powers et al. 2011) could prolong the half-life time of the PGC-1 alpha protein to compensate for the lack of mRNA induction.
The capacity of skeletal muscles for glycolysis and lipid oxygenation is increased in response to endurance exercise, which is accompanied by a higher storage potential for energy substrates, in particular carbohydrates (glycogen) and lipids (triacylglycerine). These endurance exercise-related changes in skeletal muscle structure are evoked by characteristic alterations in the transcriptional rates of various key metabolic genes (Hoppeler et al. 2011). To verify the hypothesis that the administration of antioxidants influences the expression patterns of genes of the metabolism in skeletal muscle of mice during treadmill training and in sedentary animals, we have recently quantified the mRNA levels of eight marker genes that are involved in the oxidation or exercise-dependent storage of carbohydrate and lipids, and which are known to be up-regulated in response to endurance exercise (Meier et al. 2013). As anticipated, the mRNA for each of the eight enzymes was expressed at higher levels in the tibialis anterior of trained mice than in sedentary mice. This outcome underlined the general shift of the carbohydrate and lipid metabolism in skeletal muscle towards oxidation and storage in response to endurance exercise.
While the mRNA levels of five of the eight enzymes (G6PDH, GYG, MCAD, CD36 and FABP-3) were not affected by supplemental antioxidants either in sedentary animals or with training, the concentration of HK-II, GLUT-4 and SREBF-1c mRNA was higher in the sedentary mice receiving supplemental antioxidants than in those receiving a placebo, suggesting that antioxidant supplementation at least partially mimicked the effect of endurance training on the transcription of these two genes (Figure 7.2). However, although antioxidant supplementation or endurance training each increased the expression of GLUT-4 (an important protein involved in glucose transport across the sarcolemma) to a similar degree when administered in isolation, there was no additional effect of combining the two (Meier et al. 2013). This pattern was similar for HK-II, which catalyses the phosphorylation of glucose within glycolysis (Printz et al. 1997), although in this case the effect of antioxidant supplementation alone produced the largest increase in expression (Meier et al. 2013). It has also been shown, however, that administration of vitamins C and E in isolation had no impact on GLUT-4 and HK-II expression in human skeletal muscle (Yfanti et al. 2011). However, expression of SREBF-1c, a pivotal transcription factor in biosynthesis of fatty acids (Shimano 2001), increased significantly with antioxidant supplementation or exercise alone, and to the largest degree when the two were combined (Meier et al. 2013).
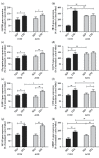
FIGURE 7.2
mRNA expression levels of eight marker genes of the carbohydrate (a through d) and the lipid (e through h) metabolism determined by real-time PCR (polymerase chain reaction). Values represent means_standard deviation from six mice for each group. Asterisks (more...)
While most studies addressing the question of whether excessive radical scavenging can actually reduce selected training stimuli by suppressing the radical-dependent signal for adaptation have been conducted at the molecular level, effects on training-induced changes in aerobic and endurance capacity have been measured in some cases. For example, daily vitamin C supplementation has been shown to greatly inhibit the peripheral adaptations to training (i.e. capillarisation and mitochondrial genesis) and mean improvement in maximal oxygen uptake has been shown to be greater (approximately double) in humans and in rats that received a placebo than in those that received vitamin C (1 g · d−1 in humans). In this study, improvement of endurance capacity (measured only in rats) was sevenfold greater (Gomez-Cabrera et al. 2008), supposedly because this parameter is more specifically related to peripheral adaptations.
Another instance of antioxidant supplementation interfering with training is when muscle injury occurs, such as after intense, unaccustomed and especially eccentric exercise. Vitamins C and E have been shown to delay healing and recovery of strength, and increase oxidative stress after such muscle-damaging exercise (Beaton et al. 2002; Childs et al. 2001; Close et al. 2006; Teixeira et al. 2009).
7.6. CONCLUSIONS
The experimental findings described above are intriguing from the standpoint of athletes and coaches who wish to improve performance capacity through training. Could it be that many are unknowingly counteracting training effectiveness through banal practices such as consuming an antioxidant-rich recovery drink after an endurance training session or taking a daily multivitamin? The issue is far from resolved. If and to what extent supplemental antioxidants are beneficial or detrimental to well-nourished athletes in training is a complex question. The answer lies, in part, in the type of training and the associated training goals.
Clearly, there are certain situations where supplementation is probably advantageous; these include high-altitude training periods, since radical production is intensified and endogenous defence weakened in hypoxia (Pialoux et al. 2006, 2009a,b), or around important competitions, where only (possible) benefits remain relevant. And of course, in the case of a diagnosed deficiency, supplementation is recommended, although this is seldom the case in healthy endurance athletes eating a balanced diet (Knez et al. 2007; Margaritis and Rousseau 2008).
In any case, it is clear considering the positive and negative effects of free radicals, and that the right balance between these and antioxidants is necessary for health and optimal training effectiveness. For the time being, identifying the optimal balance remains elusive, making the area of free radicals, antioxidants and training, especially in athletes, a ripe one for further research. Specifically, questions regarding the effects of dose (if possible, based on individual needs), timing (acutely in relation to training sessions or chronically in relation to training cycles) and setting (e.g. hypoxia) of antioxidant supplementation on RNS/ROS signalling during endurance training wait to be answered. These circumstances could be decisive in determining whether supplementation is largely beneficial or detrimental to training effectiveness. Meanwhile, few practical recommendations can be made, other than to realise that, at least for endurance athletes, antioxidant supplementation is not a case of ‘the more, the better’.
REFERENCES
- Baum O, Da Silva-Azevedo L, Willerding G. et al. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287:H2300–8. [PubMed: 15231496]
- Beaton L. J, Allan D. A, Tarnopolsky M. A, Tiidus P. M, Phillips S. M. Contraction-induced muscle damage is unaffected by vitamin E supplementation. Med Sci Sports Exerc. 2002;34:798–805. [PubMed: 11984298]
- Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and n-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–53. [PubMed: 11557312]
- Clifford P. S, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. [PubMed: 15220322]
- Close G. L, Ashton T, Cable T, Doran D, Holloway C, McArdle F, MacLaren D. P. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95:976–81. [PubMed: 16611389]
- Da Silva-Azevedo L, Jahne S, Hoffmann C. et al. Up-regulation of the peroxiredoxin-6 related metabolism of reactive oxygen species in skeletal muscle of mice lacking neuronal nitric oxide synthase. J Physiol. 2009;587:655–68. [PMC free article: PMC2670087] [PubMed: 19047200]
- Gibala M. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab. 2009;34:428–32. [PubMed: 19448710]
- Godecke A, Schrader J, Reinartz M. Nitric oxide-mediated protein modification in cardiovascular physiology and pathology. Proteom Clin Appl. 2008;2:811–22. [PubMed: 21136881]
- Gomez-Cabrera M. C, Borras C, Pallardo F. V, Sastre J, Ji L. L, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–20. [PMC free article: PMC1474177] [PubMed: 15932896]
- Gomez-Cabrera M. C, Domenech E, Romagnoli M. et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–9. [PubMed: 18175748]
- Gomez-Cabrera M. C, Domenech E, Vina J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–31. [PubMed: 18191748]
- Gross M, Baum O, Hoppeler H. Antioxidant supplementation and endurance training: Win or loss? Eur J Sport Sci. 2011;11:27–32.
- Handschin C, Spiegelman B. M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–9. [PMC free article: PMC2587487] [PubMed: 18650917]
- Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr Physiol. 2011;1:1383–412. [PubMed: 23733647]
- Hudlicka O, Brown M. D, Silgram H. Inhibition of capillary growth in chronically stimulated rat muscles by n(g)-nitro-l-arginine, nitric oxide synthase inhibitor. Microvasc Res. 2000;59:45–51. [PubMed: 10625570]
- Irrcher I, Ljubicic V, Hood D. A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–23. [PubMed: 19005163]
- Jackson M. J. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic Biol Med. 2009;47:1267–75. [PubMed: 19748570]
- Jackson M. J, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2007;102:1664–70. [PubMed: 17082364]
- Ji L. L. Antioxidant signaling in skeletal muscle: A brief review. Exp Gerontol. 2007;42:582–93. [PubMed: 17467943]
- Ji L. L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med. 2008;44:142–52. [PubMed: 18191750]
- Kanter M. Free radicals, exercise and antioxidant supplementation. Proc Nutr Soc. 1998;57:9–13. [PubMed: 9571703]
- Kayar S. R, Hoppeler H, Armstrong R. B. et al. Estimating transit time for capillary blood in selected muscles of exercising animals. Pflugers Arch. 1992;421:578–84. [PubMed: 1437519]
- Knez W. L, Jenkins D. G, Coombes J. S. Oxidative stress in half and full ironman triathletes. Med Sci Sports Exerc. 2007;39:283–8. [PubMed: 17277592]
- Kreider R. B, Almada A, Antonio J. et al. Issn exercise and sport nutrition review: Research and recommendations. Sports Nutr Rev J. 2004;1:1–44.
- Krumbach C. J, Ellis D. R, Driskell J. A. A report of vitamin and mineral supplement use among university athletes in a division i institution. Int J Sport Nutr. 1999;9:416–25. [PubMed: 10660872]
- Margaritis I, Rousseau A. S. Does physical exercise modify antioxidant requirements? Nutr Res Rev. 2008;21:3–12. [PubMed: 19079851]
- Medved I, Brown M. J, Bjorksten A. R. et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97:1477–85. [PubMed: 15194675]
- Meier P, Renga M, Hoppeler H, Baum O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem Funct. 2013;31:51–9. [PubMed: 22865599]
- Myung S. K, Ju W, Cho B, Oh S. W, Park S. M, Koo B. K, Park B. J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. Brit Med J. 2013;346:f10. [PMC free article: PMC3548618] [PubMed: 23335472]
- Padilla J, Mickleborough T. D. Does antioxidant supplementation prevent favorable adaptations to exercise training? Med Sci Sports Exerc. 2007;1887;39 author reply 88. [PubMed: 17909422]
- Pattwell D. M, McArdle A, Morgan J. E, Patridge T. A, Jackson M. J. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–72. [PubMed: 15336322]
- Pialoux V, Mounier R, Ponsot E. et al. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur J Clin Nutr. 2006;60:1345–54. [PubMed: 16788711]
- Pialoux V, Mounier R, Rock E. et al. Effects of the ‘live high-train low’ method on prooxidant/antioxidant balance on elite athletes. Eur J Clin Nutr. 2009a;63:756–62. [PubMed: 18398420]
- Pialoux V, Mounier R, Rock E. et al. Effects of acute hypoxic exposure on prooxidant/antioxidant balance in elite endurance athletes. Int J Sports Med. 2009b;30:87–93. [PubMed: 19177314]
- Powers S. K, Jackson M. J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76. [PMC free article: PMC2909187] [PubMed: 18923182]
- Powers S. K, Talbert E. E, Adhihetty P. J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–38. [PMC free article: PMC3098692] [PubMed: 21224240]
- Printz R. L, Osawa H, Ardehali H, Koch S, Granner D. K. Hexokinase ii gene: Structure, regulation and promoter organization. Biochem Soc Trans. 1997;25:107–12. [PubMed: 9056853]
- Reid M. B. Free radicals and muscle fatigue: Of ROS, canaries, and the ioc. Free Radic Biol Med. 2008;44:169–79. [PubMed: 18191753]
- Ristow M, Zarse K, Oberbach A. et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–70. [PMC free article: PMC2680430] [PubMed: 19433800]
- Sakellariou G. K, Pye D, Vasilaki A. et al. Role of superoxide–nitric oxide interactions in the accelerated age-related loss of muscle mass in mice lacking cu, zn superoxide dismutase. Aging Cell. 2011;10:749–60. [PMC free article: PMC3531889] [PubMed: 21443684]
- Shimano H. Sterol regulatory element-binding proteins (srebps): Transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439–52. [PubMed: 11591434]
- Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62:1315S–21. [PubMed: 7495226]
- Simon-Schnass I, Pabst H. Influence of vitamin E on physical performance. Int J Vitam Nutr Res. 1988;58:49–54. [PubMed: 3384584]
- Sobal J, Marquart L. F. Vitamin/mineral supplement use among athletes: A review of the literature. Int J Sport Nutr. 1994;4:320–34. [PubMed: 7874149]
- Stamler J. S, Sun Q. A, Hess D. T. A snow storm in skeletal muscle. Cell. 2008;133:33–5. [PubMed: 18394987]
- Steensberg A, Keller C, Hillig T. et al. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007;21:2683–94. [PubMed: 17470570]
- Symons M. C. Formation of radicals by mechanical processes. Free Radic Res Commun. 1988;5:131–9. [PubMed: 2853116]
- Teixeira V. H, Valente H. F, Casal S. I, Marques A. F, Moreira P. A. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med Sci Sports Exerc. 2009;41:1752–60. [PubMed: 19657294]
- Valko M, Izakovic M, Mazur M, Rhodes C. J, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. [PubMed: 15646026]
- Valko M, Leibfritz D, Moncol J, Cronin M. T, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. [PubMed: 16978905]
- Valko M, Rhodes C. J, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. [PubMed: 16430879]
- Williams M. H. Dietary supplements and sports performance: Introduction and vitamins. J Int Soc Sports Nutr. 2004;1:1–6. [PMC free article: PMC2129136] [PubMed: 18498619]
- Wray D. W, Uberoi A, Lawrenson L, Bailey D. M, Richardson R. S. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: A radically different outcome. Clin Sci (Lond). 2009;116:433–41. [PubMed: 18795893]
- Yfanti C, Nielsen A. R, Akerstrom T. et al. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Physiol Endocrinol Metab. 2011;300:E761–70. [PubMed: 21325105]
- Review Antioxidants in Athlete’s Basic Nutrition: Considerations towards a Guideline for the Intake of Vitamin C and Vitamin E.[Antioxidants in Sport Nutritio...]Review Antioxidants in Athlete’s Basic Nutrition: Considerations towards a Guideline for the Intake of Vitamin C and Vitamin E.Neubauer O, Yfanti C. Antioxidants in Sport Nutrition. 2015
- Review Acute and Chronic Effects of Antioxidant Supplementation on Exercise Performance.[Antioxidants in Sport Nutritio...]Review Acute and Chronic Effects of Antioxidant Supplementation on Exercise Performance.Bentley DJ, Ackerman J, Clifford T, Slattery KS. Antioxidants in Sport Nutrition. 2015
- Review Well-Known Antioxidants and Newcomers in Sport Nutrition: Coenzyme Q10, Quercetin, Resveratrol, Pterostilbene, Pycnogenol and Astaxanthin.[Antioxidants in Sport Nutritio...]Review Well-Known Antioxidants and Newcomers in Sport Nutrition: Coenzyme Q10, Quercetin, Resveratrol, Pterostilbene, Pycnogenol and Astaxanthin.Belviranli M, Okudan N. Antioxidants in Sport Nutrition. 2015
- Review Biomarkers Part II: Biomarkers to Estimate Bioefficacy of Dietary/Supplemental Antioxidants in Sport.[Antioxidants in Sport Nutritio...]Review Biomarkers Part II: Biomarkers to Estimate Bioefficacy of Dietary/Supplemental Antioxidants in Sport.Vassalle C, Pingitore A, De Giuseppe R, Vigna L, Bamonti F. Antioxidants in Sport Nutrition. 2015
- Review Mechanisms of Oxidative Damage and Their Impact on Contracting Muscle.[Antioxidants in Sport Nutritio...]Review Mechanisms of Oxidative Damage and Their Impact on Contracting Muscle.Kerksick CM, Zuhl M. Antioxidants in Sport Nutrition. 2015
- Supplemental Antioxidants and Adaptation to Physical Training - Antioxidants in ...Supplemental Antioxidants and Adaptation to Physical Training - Antioxidants in Sport Nutrition
Your browsing activity is empty.
Activity recording is turned off.
See more...
