NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.
Although the overall regulation of angiogenesis is dominated by metabolic factors in most tissues of the body, mechanical factors also play crucial roles in virtually every aspect of the angiogenic process. Migration of endothelial cells, tube formation (tubulogenesis), and pericyte/smooth muscle cell migration to newly formed endothelial sprouts are critical steps in the angiogenic process that depend upon mechanosensory mechanisms. These mechanosensory mechanisms need to be better understood because they represent control points in the angiogenic process that are not likely to be growth factor specific. In other words, regardless of the growth factor(s) that stimulate angiogenesis, the fundamental steps required to build new capillaries are essentially the same. A better understanding of the mechanosensory mechanisms could therefore provide the basis for unique therapeutic interventions to control angiogenesis.
4.1. Control of Blood Vessel Growth
4.1.1. Epithelial Sodium Channel Protein Biology
One possible candidate for mediating mechanosensory events in angiogenesis is the epithelial sodium channel (ENaC), which is thought to form a mechanosensory complex. ENaC proteins are members of the degenerin (DEG)/ENaC protein family, sharing amino acid homology and protein structure [254–258]. Its members include non-voltage-gated sodium channels, neurotransmitter receptors, acid sensors, and mechanosensors. Two groups of DEG/ENaC proteins identified in mammals include ENaC and acid-sensing ion channels (ASIC). ASIC proteins are activated by protons: distribution has been reported in neural tissue and sensory epithelia [259–261]. ENaC proteins are distributed widely in multiple cell types in mammals. ENaCs play a rate-limiting role in epithelial Na+ transport in kidney, lung, and colon [256–258,262–264]. They are comprised of at least our different protein subunits (α, β, γ, and δ), which are expressed in different combinations and with different subunit stoichiometries in a tissue-specific manner [257,262,263,265–267]. ENaCs are thought to require all subunits for full biologic activity; however, electrical current can be generated from channels composed of α-homomers and αβ-, αγ-, and βγ-heteromers [263,268–270]. ENaC proteins have been localized in vascular smooth muscle cells [271–278] and endothelial cells [271,279–282]: both cell types express α-, β-, and γ-subunit proteins [271–273,276,279].
4.1.2. Epithelial Sodium Channels Can Form a Mechanosensory Complex
A model of a DEG-dependent mechanosensor has been proposed to transduce mechanical forces to bioelectrical signals in nematodes. The model is based on genetic, biochemical, and functional analyses [256,257,262,264,283–285]. The mechanosensor (Figure 4.1) is thought to consist of an aqueous-filled protein channel that is tethered to the cytoskeleton and extracellular matrix (ECM), thereby allowing transmission of mechanical forces between the extracellular environment and the cell interior. The nematode mechanosensing/transducing complex may be fundamental to all metazoans, including mammals [262,285,286]. The main candidates for mechanosensors in mammals are members of the amiloride/benzamil-sensitive DEG/ENaC superfamily [254,261,287–289]: ENaC is speculated to form the aqueous-filled protein channel of the mechanosensing/transducing complex [262,285,286]. The structural nature of the channel tethers (i.e., linking proteins) is poorly understood; however, the COOH terminus of ENaC is physically and functionally linked to the cellular cytoskeleton through F-actin [290] and contributes to the control of channel activity by actin [291,292].

FIGURE 4.1
Model of mechanosensor with pore of epithelial sodium channel (ENaC) closed. ECM, extracellular matrix. Redrawn and modified after Drummond, Grifonia, and Jernigan (2008) [255].
4.1.3. Epithelial Sodium Channels Can Mediate Mechanotransduction in Mammals
ENaC family members have been shown by immunocytochemistry to be expressed in mechanoreceptor structures in the rat foot pad [293], baroreceptors [294], sensory nerve endings in rat larynx [295], sensory nerve endings of vibrissae [296], the muscle spindle [297], and vascular tissues [271–279,279–282]. Recent studies provide functional evidence that DEG/ENaCs play a role in physiological events that require mechanosensation/transduction. Shear stress can mechanically activate ENaC channels [289,298–300]. Amiloride/benzamil-sensitive ENaC channels contribute to mechanotransduction in mammalian muscle spindles [297]. Stretch-induced vasoconstriction (i.e., the myogenic response), the baroreceptor reflex, blood flow autoregulation, and migration of vascular smooth muscle cells can be attenuated using pharmacologic and/or genetic suppression of DEG/ENaC proteins [273–276,278,301–304].
4.1.4. Do Epithelial Sodium Channels Mediate Angiogenesis?
Recent studies suggest that ENaCs are required for angiogenesis [305,306]. In these studies, a specific ENaC inhibitor (benzamil) abolished both VEGF-A and FGF2 stimulated microvessel growth in the rat aortic ring angiogenesis assay [305,306]. The studies also showed that microvessel growth was reduced by about 50% in a mouse aortic ring angiogenesis assay with reduced levels of βENaC (m/m), compared with aortas from normal littermates (+/+) [306]. In these angiogenesis assays, sprouting endothelial cells interact closely with fibroblasts, macrophages, and pericytes in an orderly sequence that recapitulates all stages of angiogenesis [53,54]. The microvessels are virtually indistinguishable from capillaries that form during angiogenesis in vivo and are composed of the same cell types [52,307–309]. Endothelial cells in the explant have not been modified by repeated passages in culture and they behave like normal endothelial cells in the intact animal [54]. These recent studies [305,306] therefore support the hypothesis that ENaCs play a critical role in the angiogenic process, possibly by acting as mechanosensors for migration of endothelial and vascular smooth muscle cells as well as endothelial tube formation.
4.1.5. Physical Forces Acting on the Walls of Blood Vessels
The walls of blood vessels are subjected to mechanical forces caused by blood flow, vasodilation, and blood pressure (Figure 4.2). Blood pressure causes a cyclical mechanical strain on the walls of arteries and arterioles (where blood pressure is pulsatile) and a constant strain in capillaries and veins where blood pressure is usually nonpulsatile. Because flowing blood exhibits a viscous effect, it tends to “stick” to the endothelium creating a shear stress that is proportional to the product of fluid viscosity and the velocity gradient between adjacent layers of the flowing blood [28]. Endothelial cells in all blood vessels are exposed to shear stress, which is a force that acts tangential to the endothelial cell surface causing morphological changes to endothelial cells (Figure 4.3). The walls of arteries and veins can also be stretched circumferentially as a result of vasodilation and compressed circumferentially as a result of vasoconstriction.

FIGURE 4.2
Physical forces caused by blood flow and blood pressure act on the walls of blood vessels. Flowing blood generates shear stress tangential to the endothelial cell surface. Circumferential stretch is caused by the action of blood pressure. Redrawn after (more...)

FIGURE 4.3
Effect of laminar flow on cytoskeletal organization and orientation of endothelial cells. Cytoskeletal elements are triple stained for actin (pseudocolor blue), microtubules (green), and intermediate filaments (red). Photomicrographs were taken under (more...)
4.1.6. Shear Stress Is Sensed by the Endothelium
Molecular elements thought to play a role in sensing shear stress in endothelial cells are shown (Figure 4.4). These molecular elements include extracellular matrix (ECM), cell–ECM adhesion, cell–cell adhesion complexes, membrane components (ion channels, caveolae, surface receptors), and cytoskeletal filaments [311]. In addition, recent studies have suggested that epithelial sodium channels (ENaCs) may also play a role in sensing shear stress in multiple cell types [312–314]. Shear stress applied the luminal surface of endothelial cells is thought to be transmitted throughout the cell as well as to cell junctions and cellular adhesions to the ECM [311].
![FIGURE 4.4. Elements of shear stress mechanosensing in endothelial cells. ECM, extracellular matrix. Redrawn after Balligand, Feron, and Dessy (2009) [311].](/books/NBK53240/bin/fig4.4.gif)
FIGURE 4.4
Elements of shear stress mechanosensing in endothelial cells. ECM, extracellular matrix. Redrawn after Balligand, Feron, and Dessy (2009) [311].
4.1.7. Increased Blood Flow (Shear Stress) Can Stimulate Angiogenesis
Thoma’s [315] early observations in chick embryos that blood vessels with higher velocities of blood flow (higher shear stress) became larger whereas those with slower velocity atrophy have been substantiated in many laboratories in various animal preparations [316–320]. Mechanical factors associated with blood flow are thought to stimulate capillary development by intussusceptive angiogenesis [321,322]. In capillaries, intussusception refers to the splitting of single capillaries into two capillaries (Figure 4.5). Endothelial cells activated by shear stress [254] extend intraluminally forming two endothelial tubes through which blood can flow. Experimental proof for shear stress-induced angiogenesis has been achieved by chronic administration of vasodilators, primarily the α-adrenergic blocker, prazosin. Prolonged treatment with prazosin can increase muscle blood flow about threefold and stimulate angiogenesis [322,323–327]. Prazosin-induced angiogenesis could be VEGF-A-dependent [328]. Also, shear stress can activate the VEGFR2 pathway independent of VEGF-A [329]. Other vasodilators such as adenosine and dipyridamole (which increases adenosine levels in tissues) can also increase shear stress and stimulate angiogenesis; however, adenosine has multiple angiogenic actions independent of shear stress (Figure 3.7) [217]. Overall, the mechanism of shear stress-induced angiogenesis is poorly understood.
![FIGURE 4.5. Shear stress-induced intussusceptive angiogenesis gives rise to longitudinal splitting of blood capillaries. Redrawn after Zhou et al. (1998) [322].](/books/NBK53240/bin/fig4.5.gif)
FIGURE 4.5
Shear stress-induced intussusceptive angiogenesis gives rise to longitudinal splitting of blood capillaries. Redrawn after Zhou et al. (1998) [322].
4.1.8. Possible Role of Endothelial Cell Shape in Regulating Blood Vessel Growth and Regression
The shapes of endothelial cells can dictate their rates of growth in vitro. Cells with different shapes are generated by treating plastic cultureware with surface-active agents that alter the adherence of cells [330]. Endothelial cells with a relatively flat configuration proliferate rapidly, whereas cells with a spheroid shape grow slowly [330]. Also, DNA synthesis increases in an exponential fashion in direct relation to linear increases in cell extension [331]. Electron microscopy studies [332] have shown that vasodilation literally pulls endothelial cells into the rapidly growing flat configuration, whereas vasoconstriction compresses endothelial cells causing them to become distorted with lower growth rates (Figure 4.6).
![FIGURE 4.6. Model of endothelial cell shape during relative dilation and constriction of an arteriole. Redrawn after Stromberg et al. (1969) [332].](/books/NBK53240/bin/fig4.6.gif)
FIGURE 4.6
Model of endothelial cell shape during relative dilation and constriction of an arteriole. Redrawn after Stromberg et al. (1969) [332].
Does endothelial cell shape have a physiological role in growth regulation during long-term vasoconstriction and vasodilation? Long-term vasodilation occurs in tissues that have long-term increases in metabolic activity. The vessel growth that follows has been attributed mainly to proangiogenic growth factors released from hypoxic tissues. However, it is also possible that endothelial cell shape plays a role. Why? The flattened endothelial cells of dilated blood vessels are likely to be more susceptible to actions of angiogenic growth factors [330]. Long-term vasoconstriction can occur during the developmental stages of certain types of hypertension. Increased cardiac output leads to increased peripheral resistance (vasoconstriction) through an autoregulatory mechanism [333]. This vasoconstriction (functional rarefaction) is often followed by an actual loss of blood vessels (structural rarefaction) [140,141]. Although the structural rarefaction might be explained by overperfusion and hence overoxygenation of tissues with subsequent decreases in proangiogenic growth factors levels, it is also possible that endothelial cell shape plays a role. The compressed endothelial cells are likely to be less susceptible to the actions of proangiogenic growth factors, which would facilitate the rarefaction.
Although blood capillaries do not “vasodilate” directly, vasodilation of upstream arterioles increases capillary hydrostatic pressure and this can increase the capillary diameter and thus pull endothelial cells into a flat configuration. If this is true, and if the capillary endothelial cells release a stimulator of smooth muscle cell growth when they are pulled into a flat configuration, it could explain how capillaries that have a higher velocity of blood flow develop into larger vessels [316].
4.1.9. Mechanical Factors Have an Accessory Role in Angiogenesis
Neither blood flow nor mechanical factors associated with blood flow can actually regulate angiogenesis in heart, skeletal muscle, brain, and other tissues in which the vasculature has primary a nutritive function. Why? Because blood flow itself is regulated by metabolic factors in these tissues. For this reason, the proangiogenic actions of shear stress are thought to facilitate, but not regulate the angiogenesis. Likewise, those steps in the angiogenic process that require mechanosensation of physical stimuli serve to implement angiogenesis under the umbrella of metabolic regulation. There are, however, instances in which flow itself can be considered a controlled variable in the negative feedback regulation of vascular growth. For example, lymphangiogenesis occurs when the rate of fluid loss from blood capillaries exceeds the fluid removal capacity of resident lymph vessels. It is also possible that the flow of interstitial fluid in the interstitial spaces of the kidneys plays a role in controlling angiogenesis in the peritubular capillary bed. This latter possibility will be addressed in future editions.
4.2. CONTROL OF LYMPHANGIOGENESIS
The concept that “form follows function” also applies to the lymphatic vascular system. The main physiological function of the lymphatic system is to pump extravasated fluid and proteins from the interstitial spaces back to the blood vascular system. Fluid and proteins leak continually from the blood capillaries into the surrounding interstitial spaces. The fluid enters into lymphatic capillaries and is pumped along a series of lymphangions all the way to the venous system where the lymph is returned to the blood.
The pulmonary lymphatic system can be overwhelmed with lymph when the left atrial pressure rises too high acutely. Normally, a rise in left atrial pressure to about 40 mm Hg causes an increase in lymph flow that is insufficient to remove interstitial edema fluid from the lungs (Figure 4.7). Pulmonary edema follows. However, with slowly developing chronic pulmonary edema, the pulmonary lymphatic system can adapt to the fluid challenge by growing new lymph vessels (lymphangiogenesis) and causing existing lymph vessels to grow larger. This growth of the lymphatic system greatly increases the amount of fluid that can be removed from the lungs, thereby helping to protect against pulmonary edema.
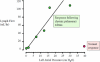
FIGURE 4.7
Lung lymph flow can increase to high levels in chronic pulmonary edema. When the lungs have excess amounts of interstitial fluid (edema) for long periods of time, lymphangiogenesis often follows. The growth of new lymph vessels or the enlargement of existing (more...)
4.2.1. Flow-Guided Lymphangiogenesis
The possibility that lymphangiogenesis can be stimulated by a long-term fluid challenge is supported by studies in the mouse tail [335]. In these experiments, a circumferential section of skin containing lymph vessels was removed from the tail, but the underlying arteries and veins remained intact. The wounded area was then wrapped with a collagen matrix providing a pathway for interstitial fluid flow and a scaffold for cell proliferation and migration. Over the next several days, lymphatic endothelial cells were found to migrate along fluid channels in the collagen matrix; they eventually formed intact lymph vessels. These findings are in contrast to “blood angiogenesis” where fluid flow occurs only after a vascular channel has been established. The investigators also found increased expression of the lymphatic endothelial cell mitogen, VEGF-C [336] in the upstream regions of the collagen bridge [335]. In other studies using a similar model (Figure 4.8), VEGF-C therapy was shown to increase lymphatic endothelial cell density in the collagen matrix, and a primary inhibition of interstitial fluid flow was found to decrease lymphatic endothelial density [337]. Together, these findings support the hypothesis that growth of the lymph vessels can be regulated by the amount of interstitial fluid to which they are challenged.

FIGURE 4.8
(A) Growth of lymphatic endothelial cells could be blocked with VEGFR3 neutralizing antibodies and rescued with VEGF-C therapy. (B) An experimental reduction in interstitial fluid (ISF) flow led to a decrease in growth of lymphatic endothelial cells. (more...)
4.2.2. High Salt Load Stimulates Lymphangiogenesis in Skin
Recent studies [338] in rats indicate that a high salt diet leads to sodium accumulation in the skin where the concentration can exceed 170 mmol/L. This high sodium concentration leads to increased density and hyperplasia of skin lymph vessels. The mechanism is thought to involve activation of tonicity-responsive enhancer binding protein (TonEBP) in macrophages that infiltrate the hypertonic environment of the skin interstitium. TonEBP binds the promoter of the gene that encodes VEGF-C increasing its secretion by the macrophages. These studies indicate that VEGF-C is an osmosensitive, hypertonically driven gene involved in lymphangiogenesis.
- Regulation: Mechanical Factors - AngiogenesisRegulation: Mechanical Factors - Angiogenesis
Your browsing activity is empty.
Activity recording is turned off.
See more...
