NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Most human pathogens that survive and proliferate in normally sterile environments have evolved specialized virulence determinants that facilitate evasion of host defense mechanisms. Microbial strategies to evade host immunity are often geared towards professional phagocytes. This is because macrophages are always on the lookout for bacteria that have breached physical barriers to gain access to a privileged site. When foreign invaders are encountered, they are usually engulfed by macrophages and then destroyed when the phagosomes in which they reside are delivered to lysosomes. To avoid destruction, many microbial pathogens modulate vesicle trafficking in eukaryotic host cells in order to prevent phagosome lysosome fusion. Although many microbial pathogens have the ability to alter phagosome trafficking, it is unclear how most of them accomplish this feat. Legionella pneumophila are bacteria that have the ability to alter maturation of the endocytic vacuole in which they reside initially, allowing them to establish an organelle within phagocytic host cells that supports replication. Rather than fusing sequentially with early endosomes, late endosomes, then lysosomes; phagosomes containing L. pneumophila will associate rapidly with smooth vesicles and are remodeled into unique organelles decorated with ribosomes. Genetic analysis has revealed that a type IV-related secretion system is required by L. pneumophila to control phagosome biogenesis. It is believed that L. pneumophila use this specialized secretion system during uptake to inject proteins into the host cell. Proteins delivered into the host cell by L. pneumophila would then act on host factors that regulate vesicle trafficking. In theory, L. pneumophila could prevent phagosome maturation by either inhibiting the function of host factors required for trafficking of endocytic vesicles or they could promote rapid remodeling of the endocytic vacuole through subverting factors used for biogenesis of other cellular organelles. Recent evidence suggests that L. pneumophila may employ a combination of both strategies. Although the effector proteins being injected into host cells have not been identified, we propose that L. pneumophila use a type-IV related secretion system to deliver one set of effectors that will inhibit endocytic maturation momentarily, and then intracellular bacteria remodel their endocytic vacuole using a second set of effectors that subvert host factors used for biosynthetic transport. This allows L. pneumophila to create a specialized organelle that shares many features with the host cell endoplasmic reticulum.
The L. pneumophila dot/icm Genes Encode a Type IVB Transporter Required for Host Cell Pathogenesis
Legionella pneumophila are gram negative bacteria that can replicate inside of eukaryotic phagocytes.1 When L. pneumophila gain access to the human lung, they can grow inside alveolar macrophages, which can result in a severe pneumonia called Legionnaires disease.24 After uptake by phagocytes, phagosomes containing L. pneumophila avoid fusion with lysosomes5 and are found intimately associated with host endoplasmic reticulum (ER).6 The ability to replicate inside of eukaryotic host cells is clearly of great importance to L. pneumophila. In nature, L. pneumophila replicate inside of fresh water protozoan hosts,7 and mutants of L. pneumophila that are unable to replicate intracellularly are avirulent in animal models of disease.810
Unlike a number of other intracellular pathogens, L. pneumophila can be cultured extracellularly on standard laboratory media11 and is relatively easy to manipulate genetically.12 These features have made L. pneumophila an excellent model organism for dissecting the molecular mechanisms that enable microbial pathogens to survive and proliferate within professional phagocytes. Towards this end several groups have independently conducted genetic screens to identify L. pneumophila mutants defective for host cell parasitism.10,1317 From these studies, it is clear that two general types of intracellular growth mutants can be isolated. The first category includes mutants that retain the ability to create a specialized phagosome that evades fusion with lysosomes. Thus, the intracellular growth defect exhibited by these mutants likely results from a genetic lesion that affects replication after formation of a protected niche. Mutants that fall into this category include those that are defective for iron utilization,18 amino acid biosynthesis, 19 nucleotide biosynthesis19 and chromosome replication.20
The key to understanding how L. pneumophila create a niche permissive for intracellular replication is held by mutant bacteria that reside in conventional phagosomes. Intracellular growth mutants of L. pneumophila that are unable to prevent endocytic maturation of their phagosomes have been identified by several laboratories.10,1315,2124 Genetic analysis of these mutants has led to the identification of 24 different genes (Fig. 1), which are clustered on two unlinked regions of the bacterial chromosome.25,26 These genes are called dot, for defect in organelle trafficking, and icm, for intracellular multiplication. L. pneumophila dot/icm mutants reside in phagosomes that can not evade fusion with lysosomes, indicating that these genes play an important role in controlling biogenesis of vacuole that supports intracellular replication.22,27,28
Significant regions of sequence similarity are found between many of the dot/icm encoded products and structural components of bacterial type IV secretion machines.25,26 These specialized protein secretion systems are found in a variety of gram negative bacteria.29 Two distinct type IV subgroups have been reported. Classification is based on sequence similarities and organization of the genes encoding the type IV transporter. The VirB proteins encoded on the Agrobacterium tumefaciens Ti plasmid are the prototypical subunits for the type IVA transporters. Type IVB transporters are defined by the Tra/Trb proteins encoded on self-transmissible plasmids such as R64 and ColIb-P9.
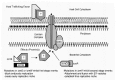
To demonstrate that the dot/icm-encoded apparatus is functionally similar to other type IV secretion systems, it was shown that L. pneumophila can mobilize plasmids containing the IncQ origin of transfer into other gram negative bacteria by a process that requires dot/icm gene function.25,26 Although these data show that DNA transfer can be mediated by the dot/icm encoded apparatus, it is important to note that transfer requires the covalent attachment of a protein molecule at the 5' terminus of the DNA strand.30 Most likely, this terminal protein is the primary substrate recognized by the transfer system, suggesting that the dot/icm-encoded apparatus can also mediate protein transfer. These data support a model in which the dot/icm genes encode a transfer system that can inject molecules from L. pneumophila into a variety of recipient cells (Fig. 2).
It was shown recently that at least 15 of the Dot/icm proteins have an orthologous Tra/Trb protein encoded on IncI1 plasmids (Fig. 1), which places the Dot/icm apparatus into the type IVB subgroup.29 It is predicted that orthologous components are playing similar roles in the two secretion systems. It has not been shown that IncI1 encoded products comprise a transporter that can alter endocytic maturation, whereas, the dot/icm-encoded apparatus is central to the ability of L. pneumophila to regulate phagosome biogenesis. Thus, these orthologous proteins are probably components required for the assembly or function of the core secretion apparatus. It is predicted that when L. pneumophila come in contact with eukaryotic host cells, the dot/icm-encoded apparatus injects proteins that can affect specific host cellular processes by either mimicking or inhibiting the function of cellular factors required for vesicle trafficking. It is reasonable to assume that the molecules conferring specialized functions to a given secretion apparatus are likely to be unique in each system. For this reason, a number of studies have focused on characterizing dot/icm proteins that do not have obvious orthologs in other secretion systems in the hope of learning more about how this secretion apparatus functions, and to identify specific factors injected into host cells by L. pneumophila that may directly affect phagosome maturation.
The Dot/Icm Transporter Plays an Essential Role in Biogenesis of a Replicative Organelle
The first L. pneumophila dot/icm mutants to be characterized in detail were those defective for a 119 kDa polytopic inner membrane protein encoded by the dotA gene. The DotA protein is similar to the ColIb-P9 protein TraY,31 suggesting that DotA is an essential component of the dot/icm transporter. Loss-of-function mutations in one of the core components of the Dot/icm transporter should eliminate all virulence processes that require this apparatus. Thus, L. pneumophila dotA mutants are predicted to be defective in secretion of all putative effector molecules that are injected into host cells by the Dot/icm transporter.
Phenotypic differences between wild type L. pneumophila and isogenic strains containing loss-of-function mutations in the dotA gene have been thoroughly analyzed in order to dissect virulence traits that require signaling through the Dot/icm transporter. From these studies, a number of interesting clues regarding the function of the Dot/icm apparatus have been revealed. First and foremost, it was shown that dotA mutants are unable to replicate inside of macrophages.32 Secondly, electron microscopy revealed that most phagosomes containing dotA mutants fuse with lysosomes, unlike compartments containing wild type L. pneumophila.22 These early data indicate that icmW function is required for bacterial evasion of lysosome fusion, and that modulation of phagosome trafficking is essential for intracellular growth.
To gain insight into how quickly L. pneumophila must alter phagosome trafficking, maturation of vesicles containing either wild type or dotA mutant bacteria was examined. Markers enriched on late endosomes, such as the protein LAMP-1, were acquired by a high percentage of phagosomes containing dotA mutant bacteria within the first 5 minutes after uptake.28 In contrast, late endosomal proteins were not observed on most phagosomes containing wild type L. pneumophila within this time frame. Furthermore, vacuoles containing wild type L. pneumophila remain LAMP-1-negative as the bacteria begin to replicate. Based on these data, it can be concluded that the Dot/icm transporter is required for a signal to be sent to host cells that alters trafficking of endocytic compartment rapidly. Most importantly, these data show that L. pneumophila actually promote bifurcation from the conventional route traveled by endocytic organelles prior to their fusion with late endosomes; thus preventing the phagosome in which these bacteria reside from fusing with lysosomes.
Several lines of evidence suggest that the Dot/icm transporter plays an important role during the initial stages of infection, but may not be required for multiplication or vacuole maintenance once a replicative organelle has been established. It is possible to phenotypically modulate Dot/icm function by taking a mutant strain of L. pneumophila harboring a defective dotA chromosomal allele and transforming this strain with a plasmid that has the functional dotA gene strictly regulated by the inducible pTAC promoter.28 The resulting strain assembles a functional Dot/icm apparatus when grown in media containing the inducing agent IPTG, whereas, bacteria grown in the absence of IPTG are defective for intracellular growth because they do not produce a functional DotA protein. When these bacteria are first grown extracellularly in broth containing IPTG, they are able to infect and grow within macrophages. As long as the tissue culture medium is supplemented with IPTG these L. pneumophila bacteria will remain phenotypically dot/icm+ as they multiply intracellularly. However, if the tissue culture medium does not include IPTG, synthesis of the DotA protein will cease upon infection. Interestingly, repressing DotA synthesis after infection does not interfere with the first complete cycle of bacterial replication in macrophages, but bacteria that exit spent host cells are phenotypically Dot/icm-negative and cannot replicate in neighboring macrophages. If these bacteria are first grown extracellularly in media that lacks IPTG, and then bacteria that are phenotypically Dot/ Icm-negative are induced with IPTG immediately after macrophage internalization, they fail to replicate intracellularly. These data suggest that trafficking decisions controlled by the Dot/Icm apparatus must be made prior to the phagosome fusing with late endocytic organelles, since activating this system after L. pneumophila have been delivered to a late endocytic compartment will not rescue intracellular growth.
Coinfection studies have provided the most convincing evidence that the Dot/icm transporter is required to create a replicative organelle, but that bacterial replication within this compartment does not require transporter function.33 When macrophages are given the opportunity to internalize both wild type L. pneumophila and dotA mutants, host cells coinfected with both strains are observed. In some of these cells, wild type and mutant bacteria reside in separate phagosomes. When this occurs, wild type L. pneumophila will replicate within the phagosomes they have created, whereas, the dotA mutants are trapped within endocytic organelles that do not permit multiplication. Coinfection can also occur when wild type and dotA mutant bacteria are internalized together, resulting in co-residence in a single phagosome. The fate of the phagosome containing both bacteria is controlled by wild type L. pneumophila. Remarkably, these compartments evade endocytic maturation and support growth of both wild type and icmW mutant bacteria. When multiplication of only those L. pneumophila encoding a functional Dot/icm transporter is selectively inhibited within co-occupied vacuoles, this can be accomplished by either using a thymine auxotroph or by antibiotic-mediated killing, growth of the dotA mutant bacteria within this same compartment does not appear to be affected. These data would suggest that once the Dot/icm apparatus has effectively established an organelle that permits intracellular multiplication, continuous signaling by this apparatus may not be necessary for expansion and maintenance of the organelle. This would be consistent with the replicative compartment being similar, if not identical, to a preexisting host organelle.
The hypothesis that L. pneumophila proteins are injected into macrophages upon contact was substantiated by evidence showing that pores are formed in the plasma membrane of host cells during their encounter with wild type bacteria.34 Mutations in the dotA gene eliminate this pore-forming activity, which demonstrates that the Dot/icm transporter is essential for this process (Fig. 2). It has been suggested that these pores represent channels in the host cell membrane through which bacterial-derived effector proteins travel.3537 There is also evidence to suggest that these pores may play an important role in the late stages of infection and mediate the egress of L. pneumophila from exhausted host cells.38 Interestingly, pore-forming activity is upregulated as L. pneumophila enter stationary phase.39 These data are consistent with both of the functions proposed for the pores since stationary phase bacteria must not only exit the host, but they must also be primed to reinitiate infection. Although a definitive role for these pores remains to be determined, pore formation has become a very useful readout for dissecting Dot/Icm transporter function.
Distinct Virulence Traits Are Regulated by Different Icm Proteins
Like most secretion systems that inject bacterial-derived proteins into eukaryotic cells, the Dot/icm apparatus has both conserved structural components predicted to be required for an operational transporter and novel factors that presumably provide functions that are unique to the L. pneumophila system. Several of these novel dot/icm-encoded products are soluble proteins, further distinguishing them from most of the conserved structural components, which have hydrophobic amino acid domains that suggest these proteins are anchored in the bacterial cell membrane (Fig. 1). To understand the role these novel proteins may play in host cell pathogenesis, the proteins icmQ, icmR, icmS, and icmW have been investigated. Distinct virulence traits that require Dot/icm transporter function have been delineated by characterizing isogenic mutants missing one of these Icm proteins.
When virulence traits were measured for icmQ, icmR, icmS and icmW mutants, three phenotypic categories were revealed. The icmQ mutant is similar in many respects to a icmW mutant.40 All virulence activities that were shown previously to require DotA protein function were also absent in an icmQ mutant. These activities include the ability to form pores in host cell membranes, create a phagosome that rapidly evades fusion with endocytic organelles, and replication in eukaryotic host cells. Thus, the icmQ protein must be essential for most, if not all, virulence activities required for host cell pathogenesis, suggesting that this protein plays an important role in Dot/icm transporter function.
Interestingly, loss-of-function mutations in either icmW or icmS will not completely abolish all virulence activities.40,41 Pore-forming activity is not affected by mutations in either icmW or icmS, however, phagosomes containing icmW or icmS mutants rapidly fuse with endocytic organelles. These data indicate that the icmW and icmS proteins are critical determinants of phagosome trafficking. Not surprisingly, the icmW and icmS mutants are totally defective for replication inside of primary macrophages derived from human and murine hosts,40,41 and are also unable to replicate inside of protozoan host cells (CRR unpublished data). A macrophage-like cell line called U937, however, will support attenuated growth of icmW and icmS mutants.4042 Limited growth in U937 cells, in combination with the observation that pore formation is not affected, indicates that icmW and icmS are not core components essential for Dot/ Icm transporter function.
The icmW and icmS proteins appear to have non-redundant functions. This was shown by constructing a mutant strain of L. pneumophila in which both the icmW and icmS genes were deleted.40 The icmW icmS double mutant is indistinguishable from either single mutant. These data indicate that the icmW and icmS proteins are required for a molecular pathway that alters endocytic trafficking of phagosomes containing L. pneumophila, which is distinct from that required for pore formation. Yeast two-hybrid analysis has revealed a specific interaction between the icmW and icmS products,40 further supporting the hypothesis that these two proteins are involved in a common signaling pathway. It seems likely that the icmW and icmS products could serve as co-chaperone proteins for substrates secreted into host cells by the Dot/icm apparatus. Accordingly, the function of secreted proteins that require icmW and icmS for injection would be to modulate phagosome biogenesis. It was shown by gel overlay analysis that the icmS protein can bind directly to a L. pneumophila protein with an estimated mass of 130,000 Daltons,40 making this 130 kDa protein an attractive candidate for an effector protein.
An unexpected phenotype was observed when the icmR gene was deleted from the L. pneumophila chromosome. Like the icmW and icmS mutants, L. pneumophila icmR mutants are still able to replicate in U937 cells, albeit inefficiently.40 Pore-forming activity, however, is not detectable in icmR mutant bacteria. Interestingly, when icmR mutants are internalized by primary murine macrophages, phagosomes in which they reside do not fuse with endocytic organelles as effectively as phagosomes containing other dot/icm mutants of L. pneumophila. As a result, a small proportion of icmR mutants that are internalized by primary macrophages can initiate replication inside of their host vacuole. After 8 hours, most vacuoles containing replicating icmR mutants have fused with lysosomes, which ends intracellular proliferation. IcmR also appears to be a chaperone protein. Gel overlay and yeast two hybrid analysis indicate that IcmQ is one protein to which IcmR binds.
From these studies, it appear as if the IcmW and IcmS proteins are essential for early phagosome biogenesis events that create a niche permissive for growth, whereas, the IcmR protein plays a critical role in establishment and maintenance of this replicative vacuole (Fig. 2). Thus, creation of a replicative organelle by L. pneumophila is a multi-stage process that requires distinct activities transduced by the Dot/icm transport apparatus.
The Dot/icm Transporter Does More than Just Inhibit Maturation of Phagosomes Containing L. pneumophila
It has been hypothesized that L. pneumophila create an inert vacuole that avoids endocytic maturation.43,44 According to this hypothesis, the primary function of the Dot/icm transporter would be to secrete molecules into the host cells that disrupt normal endocytic maturation events. To test this hypothesis we have disrupted endocytic maturation of phagosomes in primary murine macrophages by ectopically expressing dominant negative variants of the small GTP binding proteins Rab5 and Rab7 (CRR and JCK, unpublished data). During endocytic maturation, Rab5 function is required for fusion of plasma membrane-derived organelles with early endosomes4547 and Rab7 function is required for the fusion of early endosomes with late endosomal organelles.45,48,49 The dominant negative Rab5S34N protein and the dominant negative Rab7T22N protein remain locked in an inactive GDP-bound state, which disrupts the function of their wild type counterparts.47,48,5052 Expression of either Rab5S34N or Rab7T22N in primary murine macrophages will enhance the efficiency in which wild type L. pneumophila are able to form a replicative organelle (CRR and JCK, unpublished data). Close to 100 percent of all wild type L. pneumophila internalized by macrophages expressing the Rab7T22N protein will ultimately create an organelle that supports intracellular growth; whereas, only 70–75 percent of the L. pneumophila internalized by macrophages transfected with a control plasmid expressing GFP will be successful in establishing a vacuole that supports growth. These data provide proof-of-concept that the dominant interfering Rab proteins suppress the ability of macrophages to traffic phagosomes containing L. pneumophila to a compartment that restricts growth. Interestingly, L. pneumophila dot/icm mutants shown previously to replicate in vacuoles created by wild type L. pneumophila, are unable to replicate intracellularly when fed singularly to macrophages that are producing these dominant interfering Rab proteins. These findings are important because they show that simply blocking maturation of a plasma membrane-derived vacuole is insufficient to create an environment that supports L. pneumophila replication, meaning that the dot/icm transporter must be doing more for L. pneumophila than simply paralyzing the cells ability to destroy these bacteria after internalization.
Subversion of ER Vesicle Trafficking by L. pneumophila Creates a Stable Organelle that Supports Intracellular Growth
In the initial studies defining the cell biology of bacterial infection, electron micrographs taken by Horwitz and colleagues showed that phagosomes containing L. pneumophila are morphologically distinct from the endocytic vacuoles in which avirulent organisms reside (Fig. 3).6 During the first hour after infection, phagosomes containing wild type L. pneumophila are found surrounded by smooth cytoplasmic vesicles and mitochondria. As the L. pneumophila containing compartment matures, ribosomes begin to associate with the vacuole and the smooth cytoplasmic vesicles become less abundant. By eight hours, the compartment containing replicating L. pneumophila is completely surrounded by ribosomes.
In a recent study, these early morphological observations have been extended by Tilney and colleagues (Lewis J Tilney and CRR, unpublished data). These studies show that the smooth cytoplasmic vesicles reported earlier by Horwitz are actually vesicles derived from host endoplasmic reticulum (ER). ER vesicles can be seen attached to phagosomes containing L. pneumophila as early as 5 minutes after uptake. In fact, when vacuoles containing L. pneumophila are isolated from disrupted host cells, ER vesicles remain attached, indicating that this is a high affinity interaction. The plasma membrane that surrounds L. pneumophila immediately after uptake is thicker than the ER membrane. This difference in membrane thickness is likely due to the presence of cholesterol and sphingolipids, which are abundant in the plasma membrane. Remarkably, the membrane surrounding L. pneumophila becomes the same thickness as the ER membrane upon attachment of the ER vesicles, which indicates that lipid exchange occurs between these opposed vesicles. The thinning of the membrane surrounding L. pneumophila is rapid, occurring within 15–30 minutes after uptake. Most importantly, phagosomes containing L. pneumophila dotA mutants do not have attached ER vesicles and the surrounding membrane remains thick after uptake. These data indicate that the Dot/icm transporter is required for the physical interactions that occur between vacuoles harboring wild type L. pneumophila and host ER.
Analysis of other dot/icm mutants reveals that phagosomes containing icmW or icmS mutants have attached ER vesicles, whereas, icmR mutants reside in phagosomes that are not encased by ER. These data are consistent with the hypothesis that biogenesis of a replicative organelle is a multi-stage process (Fig. 4). In the first stage, phagosomes containing L. pneumophila evade immediate endocytic maturation. This stage requires the icmW and icmS gene products, as these mutants reside in phagosomes that acquire LAMP-1 rapidly by fusing with endocytic organelles. Next, L. pneumophila promote the attachment of ER-derived vesicles to the surface of their phagosomes. The IcmR protein appears to play an important role in this process. L. pneumophila icmR mutants have the ability to form phagosomes that evade fusion with lysosomes, but can not recruit ER vesicles. Data obtained in primary macrophages indicates that icmR mutants can form a vacuole that supports early replication but that eventual fusion with lysosomes severely limits intracellular proliferation. These data suggest that this second stage event, in which ER vesicles fuse with L. pneumophila containing vacuoles, is important for establishing a stable replicative niche. Once this second stage has been completed, L. pneumophila appear to be residing in a vacuole that is similar, if not identical to ER, as evidenced by the presence of ribosomes on the cytoplasmic surface of the replicative organelle. Thus, the Dot/Icm apparatus is a weapon used by L. pneumophila to beguile the host cell into converting an endocytic vacuole in which the bacteria reside initially into a cellular organelle that is nutrient rich and non-destructive.
Concluding Remarks
Although the picture of how L. pneumophila create a replicative niche is becoming clear, the molecular mechanisms used by L. pneumophila to parasitize eukaryotic hosts still remain unsolved. Identification of bacterial factors that are delivered into host cells by the Dot/icm transporter and understanding their biochemical activities will provide important clues on the host cellular machinery that is subverted during the course of infection. The fact that these factors have not been identified in the numerous genetic screens conducted previously using macrophage-like cell lines suggests that a single secreted factor may not be critical for the eventual outcome of infection. This indicates that functional redundancy probably exists among these effector molecules. In addition, a subset of these effector molecules will most likely be proteins that mimic endogenous eukaryotic factors. This means that in certain host cells, L. pneumophila may not require a given effector if the cellular equivalent can be subverted effectively. We predict that some of these effectors will have been captured from eukaryotic host cells by horizontal gene transfer. For this reason, a great deal may also be learned about how L. pneumophila has evolved as a pathogen once the substrates for the Dot/Icm secretion apparatus have been revealed.
References
- 1.
- Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. [PMC free article: PMC371671] [PubMed: 7190579]
- 2.
- McDade JE, Shepard CC, Fraser DW. et al. Legionnaires' disease: Isolation of a bacterium and demonstration of its role in other respiratory diseases. N Engl J Med. 1977;297:1197–1203. [PubMed: 335245]
- 3.
- Brenner DJ, Steigerwalt AG, McDade JE. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, family nova. Ann Intern Med. 1979;90(4):656–658. [PubMed: 434652]
- 4.
- Glavin FL, Winn WC Jr, Craighead JE. Ultrastructure of lung in Legionnaires' disease. Observations of three biopsies done during the Vermont epidemic. Ann Intern Med. 1979;90(4):555–559. [PubMed: 434634]
- 5.
- Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. [PMC free article: PMC2187157] [PubMed: 6644240]
- 6.
- Horwitz MA. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. [PMC free article: PMC2187375] [PubMed: 6619736]
- 7.
- Fields BS. The molecular ecology of Legionellae. Trends Microbiol. 1996;4(7):286–290. [PubMed: 8829338]
- 8.
- Horwitz MA. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166(5):1310–1328. [PMC free article: PMC2189638] [PubMed: 3681188]
- 9.
- Liles MR, Edelstein PH, Cianciotto NP. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol. 1999;31(3):959–970. [PubMed: 10048038]
- 10.
- Edelstein PH, Edelstein MA, Higa F. et al. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA. 1999;96(14):8190–8195. [PMC free article: PMC22210] [PubMed: 10393970]
- 11.
- Feeley JC, Gibson RJ, Gorman GW. et al. Charcoal-yeast extract agar: Primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. [PMC free article: PMC273193] [PubMed: 393713]
- 12.
- Marra A, Shuman HA. Genetics of Legionella pneumophila virulence. Annu Rev Genet. 1992;26:51–69. [PubMed: 1482124]
- 13.
- Swanson MS, Isberg RI. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64(7):2585–2594. [PMC free article: PMC174114] [PubMed: 8698483]
- 14.
- Vogel JP, Roy C, Isberg RR. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann NY Acad Sci. 1996;797:271–272. [PubMed: 8993377]
- 15.
- Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61(12):5361–5373. [PMC free article: PMC281323] [PubMed: 8225610]
- 16.
- Pope CD, Dhand L, Cianciotto NP. Random mutagenesis of Legionella pneumophila with mini-Tn10. FEMS Microbiol Lett. 1994;124(1):107–111. [PubMed: 8001761]
- 17.
- Gao LY, Harb OS, Kwaik YA. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66(3):883–892. [PMC free article: PMC107991] [PubMed: 9488371]
- 18.
- Pope CD, O'Connell W, Cianciotto NP. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun. 1996;64(2):629–636. [PMC free article: PMC173812] [PubMed: 8550218]
- 19.
- Mintz CS, Chen J, Shuman H. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988;56:1449–1455. [PMC free article: PMC259420] [PubMed: 3372016]
- 20.
- Harb OS, Abu Kwaik Y. Essential role for the Legionella pneumophila rep helicase homologue in intracellular infection of mammalian cells. Infect Immun. 2000;68(12):6970–6978. [PMC free article: PMC97806] [PubMed: 11083821]
- 21.
- Gao LY, Harb OS, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65(11):4738–4746. [PMC free article: PMC175680] [PubMed: 9353059]
- 22.
- Berger KH, Merriam JJ, Isberg RR. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. [PubMed: 7891566]
- 23.
- Andrews HL, Vogel JP, Isberg RR. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66(3):950–958. [PMC free article: PMC108001] [PubMed: 9488381]
- 24.
- Segal G, Shuman HA. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65(12):5057–5066. [PMC free article: PMC175729] [PubMed: 9393796]
- 25.
- Vogel JP, Andrews HL, Wong SK. et al. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279(5352):873–876. [PubMed: 9452389]
- 26.
- Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95(4):1669–1674. [PMC free article: PMC19142] [PubMed: 9465074]
- 27.
- Wiater LA, Dunn K, Maxfield FR. et al. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66(9):4450–4460. [PMC free article: PMC108538] [PubMed: 9712800]
- 28.
- Roy CR, Berger K, Isberg RR. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28(3):663–674. [PubMed: 9632267]
- 29.
- Christie PJ, Vogel JP. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 2000;8(8):354–360. [PMC free article: PMC4847720] [PubMed: 10920394]
- 30.
- Young C, Nester EW. Association of the virD2 protein with the 5' end of T strands in Agrobacterium tumefaciens. J Bacteriol. 1988;170(8):3367–3374. [PMC free article: PMC211303] [PubMed: 3403506]
- 31.
- Wilkins BM, Thomas AT. DNA-independent transport of plasmid primase protein between bacteria by the I1 conjugation system. Mol Microbiol. 2000;38(3):650–657. [PubMed: 11069687]
- 32.
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. [PubMed: 8382332]
- 33.
- Coers J, Monahan C, Roy CR. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nature Cell Biology. 1999;1(7):451–453. [PubMed: 10559990]
- 34.
- Kirby JE, Vogel JP, Andrews HL. et al. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27(2):323–336. [PubMed: 9484888]
- 35.
- Roy CR. Trafficking of the Legionella pneumophila phagosome. ASM News. 1999;65(6):416–421.
- 36.
- Kirby JE, Isberg RR. Legionnaires' disease: The pore macrophage and the legion of terror within. Trends Microbiol. 1998;6(7):256–258. [PubMed: 9717211]
- 37.
- Segal G, Shuman HA. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6(7):253–255. [PubMed: 9717210]
- 38.
- Alli OA, Gao LY, Pedersen LL. et al. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect Immun. 2000;68(11):6431–6440. [PMC free article: PMC97730] [PubMed: 11035756]
- 39.
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66(7):3029–3034. [PMC free article: PMC108309] [PubMed: 9632562]
- 40.
- Coers J, Kagan JC, Matthews M. et al. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38(4):719–736. [PubMed: 11115108]
- 41.
- Zuckman DM, Hung JB, Roy CR. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol. 1999;32(5):990–1001. [PubMed: 10361301]
- 42.
- Segal G, Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67(5):2117–2124. [PMC free article: PMC115946] [PubMed: 10225863]
- 43.
- Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. [PMC free article: PMC2191842] [PubMed: 7807006]
- 44.
- Clemens DL, Horwitz MA. Hypoexpression of MHC molecules on Legionella pneumophila phagosomes and phagolysosomes. Infect Immun. 1993;61:2803–2812. [PMC free article: PMC280924] [PubMed: 8514382]
- 45.
- Chavrier P, Parton RG, Hauri HP. et al. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62(2):317–329. [PubMed: 2115402]
- 46.
- Gorvel JP, Chavrier P, Zerial M. et al. rab5 controls early endosome fusion in vitro. Cell. 1991;64(5):915–925. [PubMed: 1900457]
- 47.
- Bucci C, Parton RG, Mather IH. et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70(5):715–728. [PubMed: 1516130]
- 48.
- Meresse S, Gorvel JP, Chavrier P. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci. 1995;108(Pt 11):3349–3358. [PubMed: 8586647]
- 49.
- Feng Y, Press B, Wandinger-Ness A. Rab 7: An important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131(6 Pt 1):1435–1452. [PMC free article: PMC2120682] [PubMed: 8522602]
- 50.
- Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268(32):24475–24480. [PubMed: 8226999]
- 51.
- Press B, Feng Y, Hoflack B. et al. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140(5):1075–1089. [PMC free article: PMC2132709] [PubMed: 9490721]
- 52.
- Bucci C, Thomsen P, Nicoziani P. et al. Rab7: A key to lysosome biogenesis. Mol Biol Cell. 2000;11(2):467–480. [PMC free article: PMC14786] [PubMed: 10679007]
- The L. pneumophila dot/icm Genes Encode a Type IVB Transporter Required for Host Cell Pathogenesis
- The Dot/Icm Transporter Plays an Essential Role in Biogenesis of a Replicative Organelle
- Distinct Virulence Traits Are Regulated by Different Icm Proteins
- The Dot/icm Transporter Does More than Just Inhibit Maturation of Phagosomes Containing L. pneumophila
- Subversion of ER Vesicle Trafficking by L. pneumophila Creates a Stable Organelle that Supports Intracellular Growth
- Concluding Remarks
- References
- Evasion of Phagosome Lysosome Fusion and Establishment of a Replicative Organell...Evasion of Phagosome Lysosome Fusion and Establishment of a Replicative Organelle by the Intracellular Pathogen Legionella pneumophila - Madame Curie Bioscience Database
- Approaches to Cost Recovery for Animal Research: Implications for Science, Anima...Approaches to Cost Recovery for Animal Research: Implications for Science, Animals, Research Competitiveness, and Regulatory Compliance
- Medical genetics for BioSystems (Select 1269197) (47)MedGen
Your browsing activity is empty.
Activity recording is turned off.
See more...