NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Daclatasvir is an orally available antiviral agent that inhibits the NS5A region of the hepatitis C virus (HCV) and was used in combination with other oral antiviral agents to treat chronic hepatitis C before its withdrawal in 2019. Elevations in serum enzyme levels during daclatasvir therapy are uncommon, and it has yet to be convincingly implicated in cases of idiosyncratic liver injury with jaundice. Nevertheless, successful all-oral regimens of antiviral therapy in patients with chronic hepatitis C and cirrhosis is occasionally complicated by hepatic decompensation and may cause reactivation of hepatitis B in susceptible patients coinfected with the hepatitis B virus (HBV).
Background
The hepatitis C virus is a small RNA virus that is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma in the United States as well as worldwide. Various approaches to antiviral therapy of chronic hepatitis C have been developed, starting in the 1980s with interferon alfa, which was replaced in the 1990s by long acting forms of interferon (peginterferon) to which was added the oral nucleoside analogue, ribavirin. Between 2010 and 2015, several potent oral, direct acting anti-HCV agents were developed and combinations of these found to have marked activity against the virus, allowing for highly effective therapy without use of interferon, with excellent tolerance and safety and treatment courses of 8, 12 or 24 weeks only. These direct acting agents included HCV protease (NS3/4) inhibitors, structural replication complex (NS5A) inhibitors and the HCV RNA polymerase (NS5B) inhibitors.
Daclatasvir (dak lat' as vir) is an oral antiviral agent with specific activity against the NS5A region of the hepatitis C virus. The role of the NS5A region and how daclatasvir inhibits its function are not well defined, but NS5A is necessary for formation of the replicative complex of HCV and the various NS5A inhibitors, such as daclatasvir, appear to bind to this polypeptide and prevent its participation in forming the intracellular complex that is necessary for HCV replication. In cell culture and in animal models, daclatasvir caused a rapid and marked decrease in viral replication and HCV RNA levels. In several prospective, placebo controlled trials, daclatasvir in combination with other antiviral agents (such as sofosbuvir, asunaprevir, peginterferon and ribavirin) was found to decrease HCV RNA levels and lead to sustained loss of HCV RNA in a high proportion of patients with chronic hepatitis C. Daclatasvir was approved for use in combination with other antiviral agents as therapy of chronic hepatitis C in the United States in 2015. Because of the availability of other potent HCV NS5A inhibitors combined in fixed doses with sofosbuvir (Harvoni, Epclusa) or HCV protease inhibitors (Mavyret), daclatasvir was withdrawn by the sponsor in 2019. Daclatasvir was available in the United States as tablets of 30 and 60 mg under the brand name Daklinza between 2015 and 2019. The recommended dose in adults was one capsule (60 mg) orally once daily in combination with sofosbuvir (400 mg daily) for 12 weeks. Initial indications were limited to patients with HCV genotype 3, although several studies have shown that it is also active against other HCV genotypes. Side effects were uncommon, but were generally mild and included headache, fatigue and nausea.
Hepatotoxicity
In large randomized controlled trials, daclatasvir was not associated with serum enzyme elevations during therapy. A difficulty in assessing side effects of daclatasvir and other anti-HCV agents, however, was that they are never used as monotherapy, but were also combined with agents active against other HCV targets, such as the viral protease (NS3) or polymerase (NS5B). Daclatasvir was also commonly used in combination with the more traditional agents used for hepatitis C, such as peginterferon and ribavirin, both of which have prominent adverse effects. In combination with asunaprevir (an HCV protease inhibitor), daclatasvir was associated with serum ALT elevations in 3% to 11% of patients and with several instances of acute hypersensitivity and hepatitis, some of which were severe. The cause of the hypersensitivity reaction, however, appeared to be asunaprevir. In combination with sofosbuvir, daclatasvir was not associated with serum enzyme elevations or with clinically apparent liver injury.
Daclatasvir has, however, been implicated in rare instances of acute decompensation of HCV related cirrhosis. The role of daclatasvir and the other HCV antivirals in this syndrome, however, was unclear. The liver injury usually arose within 2 to 6 weeks of starting therapy, but occasionally later and even after discontinuation of therapy. The injury was marked by worsening jaundice and signs of hepatic failure. In some instances, lactic acidosis was present early. In most but not all instances, the serum enzymes increased minimally if at all, despite the worsening hepatic failure. Several instances resulted in death or need for emergency liver transplantation. For this reason, it was recommended that patients with cirrhosis undergoing antiviral therapy with potent direct acting agents should be monitored carefully, particularly during the first few weeks of treatment.
Finally, reactivation of hepatitis B has occurred in rare patients being treated for chronic hepatitis C some of whom had received daclatasvir. The relationship of HBV reactivation to the antiviral treatment of HCV infection is not clear, but it may be due to clearance of HCV replication which allows HBV DNA levels to increase.
Likelihood score: C (probable rare cause of clinically apparent liver injury in patients with cirrhosis or preexisting hepatitis B virus coinfection).
Mechanism of Injury
The mechanism by which daclatasvir might cause liver injury is not known. It is metabolized in the liver largely via the cytochrome P450 system, predominantly CYP 3A4 and liver injury may be due to production of a toxic or immunogenic metabolite. Daclatasvir is also susceptible to drug-drug interactions with strong CYP 3A4 inducers (such as efavirenz or rifampin) or inhibitors (such as ketoconazole).
Outcome and Management
Daclatasvir in combination with other potent direct acting agents for hepatitis C (such as sofosbuvir) has been associated with instances of hepatic decompensation in patients with preexisting cirrhosis. For these reasons, monitoring of liver tests is recommended, particularly during the first 4 weeks of therapy. Treatment should be interrupted with jaundice and signs of hepatic decompensation arise. All patients who are to receive antiviral therapy for hepatitis C should be screened for presence of hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc). Those who are reactive for the HBV markers should be monitored for evidence of reactivation during antiviral therapy of hepatitis C with serial testing for HBV DNA and started on therapy for hepatitis B if HBV DNA levels appear de novo or rise significantly.
Drug Class: Antiviral Agents, Hepatitis C Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Daclatasvir – Daklinza®
DRUG CLASS
Hepatitis C Agents
Product labeling at DailyMed, National Library of Medicine, NIH
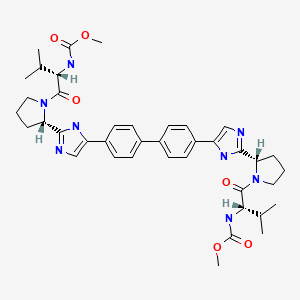
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Daclatasvir | 1009119-64-5 | C40-H50-N8-O6 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 07 February 2022
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss oral, direct acting antiviral agents used to treat hepatitis C).
- Lemm JA, Leet JE, O'Boyle DR 2nd, Romine JL, Huang XS, Schroeder DR, Alberts J, et al. Discovery of potent hepatitis C virus NS5A inhibitors with dimeric structures. Antimicrob Agents Chemother. 2011;55:3795–802. [PMC free article: PMC3147613] [PubMed: 21576451](High throughput screening of molecules for activity in a HCV replicon system identified a thiazolidinone NS5A inhibitor that was characterized as a dimer molecule, a derivative of which was synthesized leading to the identification of a stable, active agent, BMS-790052 [daclatasvir], which was then shown to have excellent clinical activity).
- Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–24. [PubMed: 22256805](Among 21 previously treated patients with chronic hepatitis C, genotype 1, who received daclatasvir and asunaprevir with or without peginterferon and ribavirin, 4 of 9 [44%] who received the oral antiviral agents alone had an SVR and 6 of the 21 had transient ALT elevations above 3 times ULN [peak: 370 U/L], but all resolved and none were associated with jaundice).
- Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hézode C, et al. Daclatasvirr for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671–7. [PubMed: 22714001](Among 48 patients treated with peginterferon and ribavirin in combination with placebo or one of 3 doses of daclatasvir for 48 weeks, SVR rates were higher with daclatasvir [42% to 92%] than placebo [25%] and adverse events were similar in all groups).
- Sheridan C. Calamitous HCV trial casts shadow over nucleoside drugs. Nat Biotechnol. 2012;30:1015–6. [PubMed: 23138280](News report on cases of cardiotoxicity associated with an HCV RNA polymerase inhibitor [BMS 986094; a pro-drug of 6-O-methyl-2’-C-methylguanosine] combined with daclatasvir).
- Suzuki Y, Ikeda K, Suzuki F, Toyota J, Karino Y, Chayama K, Kawakami Y, et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58:655–62. [PubMed: 23183526](Among 43 patients with chronic hepatitis C, genotype 1b, treated with daclatasvir and asunaprevir alone for 24 weeks, 77% had an SVR; 12 patients had ALT elevations, 2 of whom developed jaundice, but the liver injury resolved in all, although 4 required early discontinuation).
- Everson GT, Sims KD, Rodriguez-Torres M, Hézode C, Lawitz E, Bourlière M, Loustaud-Ratti V, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146:420–9. [PubMed: 24184132](Among 66 patients with chronic hepatitis C, genotype 1, treated with daclatasvir, asunaprevir and a nonnucleoside NS5B inhibitor for 12 or 24 weeks, 61 had an SVR and none had ALT elevations above 5 times ULN).
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, et al. AI444040 Study Group. Daclatasvirr plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21. [PubMed: 24428467](Among 211 patients with chronic hepatitis C treated with daclatasvir and sofosbuvir with or without ribavirin for 12 or 24 weeks, SVR rates were 98% for genotype 1, 92% genotype 2 and 89% genotype 3; serious adverse events occurred in 10 patients, but none were liver related, and there was no mention of ALT elevations).
- Lok AS, Gardiner DF, Hézode C, Lawitz EJ, Bourlière M, Everson GT, Marcellin P, et al. Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders. J Hepatol. 2014;60:490–9. [PubMed: 24444658](Among 101 patients with chronic hepatitis C treated with a 24 week course of daclatasvir with various regimens including asunaprevir, peginterferon and ribavirin, 4 patients [4%] had ALT elevations above 5 times ULN [peak: 590 U/L], but none had symptoms or jaundice and all recovered without need of early discontinuation).
- Belema M, Nguyen VN, Bachand C, Deon DH, Goodrich JT, James CA, Lavoie R, et al. Hepatitis C virus NS5A replication complex inhibitors: the discovery of daclatasvir. J Med Chem. 2014;57:2013–32. [PubMed: 24521299](Description of the identification of an inhibitor of the NS5A replication complex and subsequent further refinement of the compound to allow for oral absorption: compound 33, daclatasvir).
- Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, et al. Daclatasvirr plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–91. [PMC free article: PMC4315868] [PubMed: 24604476](Among 222 Japanese patients with chronic hepatitis C, genotype 1, treated with daclatasvir and asunaprevir for 24 weeks, 85% achieved an SVR, while ALT elevations above 5 times ULN occurred in 16 patients [7%], two with concurrent bilirubin elevations, 10 requiring early discontinuation; enzyme elevations arose within 4 to 23 weeks of starting and resolved within 2 to 3 weeks of stopping).
- Pellicelli AM, Montalbano M, Lionetti R, Durand C, Ferenci P, D'Offizi G, Knop V, et al. Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig Liver Dis. 2014;46:923–7. [PubMed: 24997638](Among 12 patients with severe chronic hepatitis C, genotype 1 and 4, after liver transplantation treated with sofosbuvir and daclatasvir with or without ribavirin for 24 weeks, 3 died of progressive hepatic failure during therapy [weeks 4, 8 and 10] and the other 9 had an SVR).
- Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, et al. HALLMARK-DUAL Study Team. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–605. [PubMed: 25078304](Among 747 patients with chronic hepatitis C, genotype 1b, treated with daclatasvir and asunaprevir or placebo for 12 or 24 weeks, 21 patients [3%] had ALT elevations above 5 times ULN and 7 [1%] were withdrawn from treatment early because of ALT elevations).
- Fujii Y, Uchida Y, Mochida S. Drug-induced immunoallergic hepatitis during combination therapy with daclatasvir and asunaprevir. Hepatology. 2015;61:400–1. [PubMed: 25308083](57 year old Japanese man with chronic hepatitis C, genotype 1b, developed fever and eosinophilia at week 2 and jaundice by week 4 of therapy with daclatasvir and asunaprevir [bilirubin 3.3 mg/dL, ALT 609 U/L, Alk P 320 U/L], resolving rapidly with stopping therapy and starting prednisone, HCV relapsing in follow up).
- Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, et al. ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35. [PMC free article: PMC4409820] [PubMed: 25614962](Among 152 patients with chronic hepatitis C, genotype 3, treated with sofosbuvir and daclatasvir for 12 weeks, 135 [89%] had an SVR, and there were no treatment related serious adverse events, early discontinuations for adverse events or de novo ALT elevations above 5 times ULN).
- Jensen D, Sherman KE, Hézode C, Pol S, Zeuzem S, de Ledinghen V, Tran A, et al. HALLMARK-QUAD Study Team. Daclatasvirr and asunaprevir plus peginterferon alfa and ribavirin in HCV genotype 1 or 4 non-responders. J Hepatol. 2015;63:30–7. [PubMed: 25703086](Among 398 previously treated patients with chronic hepatitis C, genotype 1 or 4, treated with daclatasvir, asunaprevir, peginterferon and ribavirin for 24 weeks, SVR rates were 93% and 100%; ALT elevations above 5 times ULN occurred in 12 patients [3%], one with concurrent elevation in bilirubin, arising within 16 to 86 days of starting and resolving within 9 to 85 days).
- Cornella SL, Stine JG, Kelly V, Caldwell SH, Shah NL. Persistence of mixed cryoglobulinemia despite cure of hepatitis C with new oral antiviral therapy including direct-acting antiviral sofosbuvir: A case series. Postgrad Med. 2015;127:413–7. [PubMed: 25746436](Among 5 patients with chronic hepatitis C and cryoglobulinemia, successful therapy with antiviral regimens resulted in loss of cryoglobulins in only 2 patients, and most had persistence of renal and neurologic symptoms despite clearance of HCV RNA and resolution of liver disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiviral agents, but all were antiretroviral agents and no case was attributed to the oral direct acting agents used to treat hepatitis C).
- In brief: severe bradycardia with sofosbuvir and amiodarone. Med Lett Drugs Ther. 2015;57(1466):58. [PubMed: 25853664](Summary of FDA warning regarding life threatening bradycardia in patients on amiodarone therapy who are treated with sofosbuvir, occurring within hours to up to 12 days after starting; 3 patients required pacemakers and one died).
- Muir AJ, Poordad F, Lalezari J, Everson G, Dore GJ, Herring R, Sheikh A, et al. Daclatasvirr in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA. 2015;313:1736–44. [PubMed: 25942724](Among 202 patients with chronic hepatitis C, genotype 1, and cirrhosis treated with daclatasvir, asunaprevir and beclabuvir with or without ribavirin for 12 weeks, SVR rates were 87% to 98%; ALT elevations above 5 times ULN occurred in 4 patients [2%], one with concurrent bilirubin elevations [bilirubin 2.4 mg/dL, ALT 992 U/L], which resolved within 6 weeks of early discontinuation).
- Bunchorntavakul C, Reddy KR. Review article: the efficacy and safety of daclatasvir in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2015;42:258–72. [PubMed: 26014906](Review of safety and efficacy of daclatasvir for chronic hepatitis C, mentions that 5% to 29% of patients treated with both daclatasvir and asunaprevir developed significant ALT elevations, but these were most likely due to asunaprevir).
- Leroy V, Dumortier J, Coilly A, Sebagh M, Fougerou-Leurent C, Radenne S, Botta D, et al.; Agence Nationale de Recherches sur le SIDA et les Hépatites Virales CO23 Compassionate Use of Protease Inhibitors in Viral C in Liver Transplantation Study Group. Efficacy of sofosbuvir and daclatasvir in patients with fibrosing cholestatic hepatitis C after liver transplantation. Clin Gastroenterol Hepatol. 2015;13:1993-2001.e1-2. [PubMed: 26044317](Among 23 patients with fibrosing cholestatic hepatitis C after liver transplantation treated with sofosbuvir and daclatasvir [n=15] or sofosbuvir and ribavirin [n=8] for 24 weeks, SVR was achieved in 22 [96%]; one patient developed worsening cholestasis after 12 weeks that did not improve when daclatsavir was stopped [16 weeks], but did when sofosbuvir was stopped [24 weeks]).
- Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, Farley MM, Hepatitis B. Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clin Infect Dis. 2015;61(8):1304–6. [PubMed: 26082511](2 cases: 55 year old man with chronic hepatitis C, genotype 1, and HBsAg with low levels of HBV DNA [2300 IU/mL] developed jaundice 8 weeks after starting sofosbuvir and simeprevir [bilirubin 12.2 mg/dL, ALT 1495 U/L, INR 1.96, HBV DNA 22 million IU/mL], with resolution within 6 weeks of stopping HCV agents and starting tenofovir and emtricitabine [Case 2]; 57 year old man with chronic hepatitis C, genotype 1a, and anti-HBc without HBsAg developed rising levels of HBV DNA during therapy with sofosbuvir and simeprevir [Pre <20, 2 weeks 353 and 4 weeks 11,255 IU/mL], which fell to undetectable levels within 8 weeks of starting tenofovir with emtricitabine [Truvada], ALT values remaining normal during the episode).
- Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–22. [PubMed: 26100497](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis, suggests that genotype 1 infected patients should receive an all-oral regimen such as dual therapy with sofosbuvir and ledipasvir or daclatasvir or the triple combination of dasabuvir with ombitasvir and paritaprevir, the major unresolved issues being duration of therapy and the role of ribavirin).
- Kalafateli M, Dusheiko G, Manousou P. Clinical decompensation after achieving SVR with sofosbuvir, daclatasvir and ribavirin in a patient with recurrent HCV post-liver transplant. J Gastrointestin Liver Dis. 2015;24:257–8. [PubMed: 26114189](33 year old male with hemophilia and chronic hepatitis C, genotype 3, underwent liver transplantation and had recurrence of HCV posttransplant, subsequently failing to respond to several interferon based courses of therapy, eventually responding to sofosbuvir, daclatasvir and ribavirin, but then developing hepatic decompensation 2 months after achieving an SVR).
- Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–23. [PubMed: 26159282](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis summarizing the high rate of adverse events, including hepatic decompensation and death with peginterferon based regimens combined with boceprevir or telaprevir and the more effective and better tolerated all oral regimens).
- Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, Sherman KE, et al. ALLY-2 Investigators. Daclatasvirr plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714–25. [PubMed: 26196502](Among 203 patients with chronic hepatitis C, genotype 1 to 4, and HIV coinfection treated with sofosbuvir and daclatasvir for 8 or 12 weeks, SVR rates were 97% to 98% [12 weeks] and 76% [8 weeks] and there were no discontinuations for adverse events, treatment related serious adverse events or ALT elevations above 3 times ULN).
- Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164. [PMC free article: PMC4535371] [PubMed: 26215390](59 year old woman with chronic hepatitis C, genotype 1, and anti-HBc without HBsAg or HBV DNA in serum, developed jaundice 11 weeks after starting sofosbuvir and simeprevir [bilirubin 9.1 mg/dL, ALT 2263 U/L, INR 1.9, HBV DNA 29 million IU/mL]; she was started on tenofovir, but developed progressive liver failure and underwent emergency liver transplantation 10 days after presentation).
- Shibata S, Umemura T, Komatsu M, Tanaka E. Severe hepatotoxicity associated with asunaprevir and daclatasvir in chronic hepatitis C. Hepatology. 2016;63:2063–4. [PubMed: 26248724](58 year old Japanese woman with chronic hepatitis C, genotype 1b, developed fever followed by dark urine 11 days after starting daclatasvir and asunaprevir [bilirubin 6.6 mg/dL, ALT 114 U/L, Alk P 253 U/L, eosinophils 20%], with severe course [ascites and coagulopathy], but eventual full recovery).
- Renet S, Chaumais MC, Antonini T, Zhao A, Thomas L, Savoure A, Samuel D, et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology. 2015;149:1378–80.e1. [PubMed: 26253303](Two patients taking amiodarone developed severe bradycardia within 2 hours of receiving sofosbuvir and daclatasvir, one of whom had a positive rechallenge 13 days, but not 8 weeks after stopping amiodarone).
- Kumada H, Suzuki F, Suzuki Y, Toyota J, Karino Y, Chayama K, Kawakami Y, et al. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese HCV patients. J Gastroenterol Hepatol. 2016;31:14–22. [PubMed: 26252875](Among 252 patients with chronic hepatitis C, genotype 1, treated with daclatasvir and asunaprevir vs telaprevir and peginterferon with ribavirin, SVR rates were higher [89% vs 62%] and adverse event rates lower with the all oral regimen, but one patient discontinued therapy early because of ALT elevations above 5 times ULN, resolving on stopping and achieving an SVR; ALT elevations occurred in 17% of those receiving daclatasvir and asunaprevir and were above 5 times ULN in 11%).
- Fujii Y, Uchida Y, Mochida S. Reply to the Letter Entitled "Severe Hepatotoxicity Associated with Asunaprevir and Daclatasvirr in Chronic Hepatitis C". Hepatology. 2016;63:2064–5. [PubMed: 26248724](Letter in response to Shibata [2015] mentions that immunoallergic liver injury occurred in 9 of 297 patients treated with asunaprevir and daclatasvir with fever, CRP elevations or eosinophilia arising 4 to 20 weeks after starting therapy).
- Fontana RJ, Brown RS, Moreno-Zamora A, Prieto M, Joshi S, Londoño MC, et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl. 2016;22(4):446–58. [PubMed: 26890629](Among 97 liver transplant recipients treated with daclatasvir and either sofosbuvir or simeprevir with or without ribavirin for up to 24 weeks, 84 [87%] had a sustained response and 8 [8%] patients died, but none of the deaths were considered due to the antiviral therapy).
- Suda G, Kudo M, Nagasaka A, Furuya K, Yamamoto Y, Kobayashi T, Shinada K, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016 Jul;51(7):733–40. [PubMed: 26768604](Among 21 patients with chronic hepatitis C, genotype 1, and renal failure on hemodialysis who were treated with daclatasvir and asuneprevir for 24 weeks, 20 [96%] had a sustained response, including 1 patient who stopped treatment at week 12 because of ALT elevations 10 times ULN).
- Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir With Sofosbuvir and Ribavirin for HCV Infection With Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology. 2016;63(5):1493–505. [PMC free article: PMC5069651] [PubMed: 26754432](Among 60 patients with advanced cirrhosis and 53 transplant recipients with chronic hepatitis C who were treated with daclatasvir, sofosbuvir and ribavirin for 12 weeks, sustained response rates were 93% in Child-Pugh class A and B cirrhosis and 56% in class C, and 95% in transplant patients; " there were no treatment related serious adverse events").
- Kao JH, Jensen DM, Manns MP, Jacobson I, Kumada H, Toyota J, Heo J, et al. Daclatasvir plus asunaprevir for HCV genotype 1b infection in patients with or without compensated cirrhosis: a pooled analysis. Liver Int. 2016;36(7):954–62. [PubMed: 26683763](In a pooled analysis of 4 clinical trials of daclatasvir and asunaprevir, sustained response rates were similar in those with and without cirrhosis [84%and 84%], serious adverse events were less common [1% vs 3%], and there were no deaths).
- Jafri SM, Gordon SC. The safety of daclatasvir for the treatment of hepatitis C. Expert Opin Drug Saf. 2015;14:1787–97. [PubMed: 26571362](Survey of the literature on safety of daclatasvir concludes that it is effective).
- Hézode C, Alric L, Brown A, Hassanein T, Rizzetto M, Buti M, et al. COMMAND-4 study team. Randomized controlled trial of the NS5A inhibitor daclatasvir plus peginterferon and ribavirin for HCV genotype-4 (COMMAND-4). Antivir Ther. 2015;21:195–205. [PubMed: 26313445](Among 124 patients with chronic hepatitis C, genotype 4, treated with daclatsavir or placebo with peginterferon and ribavirin for 24 to 48 weeks, sustained response rates were 82% [with daclatasvir] and 43% [without] and there were no serious adverse events attributed to daclatasvir).
- European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. [PubMed: 25911336](Guidelines for the antiviral therapy of chronic hepatitis C from the European liver disease research and academic society).
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. [PubMed: 26111063](Guidelines for the antiviral therapy of chronic hepatitis C from the US liver and infectious diseases research and academic societies).
- Daclatasvir (Daklinza) for hepatitis C virus genotype 3. Med Lett Drugs Ther. 2015;57(1479):142–3. [PubMed: 26445205](Concise review of the mechanism of action, efficacy, safety and costs of daclatasvir as therapy of hepatitis C, does not mention hepatotoxicity or ALT elevations).
- Everson GT, Sims KD, Thuluvath PJ, Lawitz E, Hassanein T, Rodriguez-Torres M, et al. Daclatasvir + asunaprevir + beclabuvir ± ribavirin for chronic HCV genotype 1-infected treatment-naive patients. Liver Int. 2016;36:189–97. [PubMed: 26473667](Among 187 patients with chronic hepatitis C, genotype 1, who were treated with daclatasvir, asunaprevir, beclabuvir with or without ribavirin for 12 weeks, 169 [90%] had a sustained response, one patient had a transient elevation in ALT [114 U/L] and AST [295 U/L] without symptoms or jaundice, which resolved without dose modification).
- Akuta N, Sezaki H, Suzuki F, Kawamura Y, Hosaka T, Kobayashi M, Kobayashi M, et al. Relationships between serum asunaprevir concentration and alanine aminotransferase elevation during daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. J Med Virol. 2016;88:506–11. [PubMed: 26292191](Among 70 patients with chronic hepatitis C, genotype 1b, treated with daclatasvir and asunaprevir who had serum levels measured, ALT elevations were more frequent in those with higher serum levels of asunaprevir).
- Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234–8. [PubMed: 26325535](36 year old woman with HCV related cirrhosis developed worsening hepatic decompensation within 3 weeks of starting sofosbuvir, daclatasvir and ribavirin [peak bilirubin 30.5 mg/dL, ALT 96 U/L, Alk P 398 U/L] and she continued to worsen after antiviral therapy was stopped, undergoing successful liver transplantation on day 37 after starting).
- Marchan-Lopez A, Dominguez-Dominguez L, Kessler-Saiz P, Jarrin-Estupiñan ME. Liver failure in human immunodeficiency virus - hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy. J Hepatol. 2016;64:752–3. [PubMed: 26682727](Letter in response to Dyson [2016]: 49 year old man with chronic hepatitis C, cirrhosis [Child-Pugh class B] and HIV coinfection developed worsening hepatic decompensation 1 to 2 months after starting sofosbuvir and ledipasvir that worsened for two weeks after stopping [peak bilirubin 46.9 mg/dL, INR 3.17], and then resolved; he later tolerated reinitiation of antiretroviral drugs).
- Dyson JK, McPherson S. Reply to "Liver failure in human immunodeficiency virus - Hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy". J Hepatol. 2016;64:753–4. [PubMed: 26682725](Letter in reply to March-Lopez [2016] reporting another case of hepatic decompensation during sofosbuvir, ledipasvir and ribavirin therapy of a patient hepatitis C, cirrhosis and HIV coinfection, arising within 6 weeks of starting treatment [bilirubin 12.6 mg/dL, protime 17 sec], and leading to successful, emergency liver transplantation).
- Welker MW, Luhne S, Lange CM, Vermehren J, Farnik H, Herrmann E, Welzel T, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/ sofosbuvir treatment. J Hepatol. 2016;64:790–9. [PubMed: 26658684](Among 35 patients with chronic hepatitis C and advanced fibrosis or cirrhosis treated with sofosbuvir based regimens, 12 [34%] had a serious adverse event and 5 [14%] developed lactic acidosis, largely in those with Child-Pugh class B and C cirrhosis and in the context of hepatic decompensation, 2 of whom died).
- Hoofnagle JH. Hepatic decompensation during direct-acting antiviral therapy of chronic hepatitis C. J Hepatol. 2016;64:763–5. [PubMed: 26795828](Editorial in response to Welker [2016] discussing the occurrence of unexplained hepatic decompensation during antiviral therapy of hepatitis C and whether these episodes are coincidental, caused by hepatoxicity of the antiviral drugs, or are the paradoxical result of sudden eradication of the chronic viral infection).
- Miyashima Y, Honma Y, Miyagawa K, Oe S, Senju M, Shibata M, Hiura M, et al. Daclatasvir and asunaprevir combination therapy-induced hepatitis and cholecystitis with coagulation disorder due to hypersensitivity reactions. Intern Med. 2016;55:3595–601. [PMC free article: PMC5283959] [PubMed: 27980259](70 year old woman developed skin rash, fever and abdominal tenderness with minimal change in bilirubin and serum enzymes 2 weeks after starting daclatasvir and asunaprevir for chronic hepatitis C [bilirubin 1.1 mg/dL, ALT 102 U/L, Alk P 220 U/L, eosinophils 13%], resolving within 6 weeks of stopping).
- Morio K, Imamura M, Kawakami Y, Morio R, Kobayashi T, Yokoyama S, Nagaoki Y, et al. Real-world efficacy and safety of daclatasvir and asunaprevir therapy for hepatitis C virus-infected cirrhosis patients. J Gastroenterol Hepatol. 2017;32:645–50. [PubMed: 27513614](Among 252 Japanese patients with chronic hepatitis C treated with daclatasvir and asunaprevir for 24 weeks, the SVR rate was 93%, ALT elevations above 3 times ULN occurred in 17 [7%; discontinuation because of ALT elevations in 2 [1%] and liver decompensation in 1).
- Benítez-Gutiérrez L, de Mendoza C, Baños I, Duca A, Arias A, Treviño A, Requena S, et al. Drug-induced lung injury in a liver transplant patient treated With sofosbuvir. Transplant Proc. 2016;48:2515–8. [PubMed: 27742338](Among 24 liver transplant recipients with chronic hepatitis C treated with sofosbuvir containing antiviral regimens, 23 [95%] achieved an SVR, but one developed severe respiratory failure [suspected drug induced lung injury] 10 days after starting therapy, which was successfully treated with prednisone and she was later was successfully treated with 24 weeks of daclatasvir and simeprevir and achieved an SVR).
- Suda G, Nagasaka A, Yamamoto Y, Furuya K, Kumagai K, Kudo M, Terashita K, et al. NORTE Study Group. Safety and efficacy of daclatasvir and asunaprevir in hepatitis C virus-infected patients with renal impairment. Hepatol Res. 2017;47:1127–36. [PubMed: 27943523](Among 322 patients with renal dysfunction and chronic hepatitis C treated with daclatasvir and asunaprevir for 24 weeks, 289 [90%] achieved an SVR, 17 [5%] had ALT elevations above 3 times ULN, of whom 8 discontinued therapy early, 7 of whom achieved an SVR and no patient developed clinically apparent liver injury).
- Suda G, Kudo M, Nagasaka A, Furuya K, Yamamoto Y, Kobayashi T, Shinada K, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016;51:733–40. [PubMed: 26768604](Among 21 patients with chronic hepatitis C and renal failure on hemodialysis treated with daclatasvir and asunaprevir for 24 weeks, 20 [95%] had an SVR and 3 [14%] had ALT elevations above 5 times ULN, one of whom discontinued therapy early, but still achieved an SVR).
- Pol S, Bourliere M, Lucier S, Hezode C, Dorival C, Larrey D, Bronowicki JP, et al. ANRS/AFEF HEPATHER study group. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J Hepatol. 2017;66:39–47. [PubMed: 27622858](Among 798 patients with chronic hepatitis C, genotype 1, [80% with cirrhosis] treated with sofosbuvir and daclatasvir with or without ribavirin for 12 or 24 weeks, the overall SVR rate was 95%, cirrhotic patients having a higher SVR rate with 24 than 12 weeks [96% vs 92%]; two patients with cirrhosis died of end-stage liver disease at 11 and 24 weeks of treatment).
- Lawitz E, Poordad F, Gutierrez JA, Kakuda TN, Picchio G, Beets G, Vandevoorde A, et al. Simeprevir, daclatasvir and sofosbuvir for hepatitis C virus-infected patients with decompensated liver disease. J Viral Hepat. 2017;24:287–294. [PubMed: 27878906](Among 40 patients with chronic hepatitis C, genotype 1 or 4, and cirrhosis treated with sofosbuvir, simeprevir and daclatasvir for 12 weeks, all patients achieved an SVR and while 2 patients had an episode of decompensation on treatment and Child’s Pugh scores worsened in 7 patients, there were no deaths and no early discontinuations).
- Hézode C, Almasio PL, Bourgeois S, Buggisch P, Brown A, Diago M, Horsmans Y, et al. Simeprevir and daclatasvir for 12 or 24 weeks in treatment-naïve patients with hepatitis C virus genotype 1b and advanced liver disease. Liver Int. 2017;37:1304–1313. [PubMed: 28135777](Among 106 adults with chronic hepatitis C, genotype 1, and advanced liver disease treated with simeprevir and daclatasvir for 12 or 24 weeks, the overall SVR rate was 92% with both 12 and 24 weeks of treatment, 70% had adverse events, 6% serious adverse events but there was only one severe liver related event, a transient and asymptomatic rise in ALT and AST levels above 5 times ULN).
- Hézode C, Lebray P, De Ledinghen V, Zoulim F, Di Martino V, Boyer N, Larrey D, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int. 2017;37:1314–1324. [PMC free article: PMC5600115] [PubMed: 28177199](Among 333 adults with chronic hepatitis C, genotype 3, [cirrhosis in 77%] treated with sofosbuvir and daclatasvir for 24 weeks, the overall SVR rate was 89%, and was 88% in those with and 98% in those without cirrhosis, while the adverse event rate was 37%, serious adverse event rate 14%, 3 patients developed decompensation during treatment and one died of liver failure).
- Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, Sulkowski MS, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66(4):1090–1101. [PMC free article: PMC5756478] [PubMed: 28504842](Among 412 patients with liver or kidney transplants or both and chronic hepatitis C treated with direct acting antiviral agents with or without ribavirin in a prospective observational study, the SVR rate was 96% with sofosbuvir and ledipasvir and somewhat lower with sofosbuvir and daclatasvir and Viekira Pak; serious adverse events occurred in 10%, including hepatic encephalopathy in 2 patients).
- El-Khayat H, Fouad Y, Mohamed HI, El-Amin H, Kamal EM, Maher M, Risk A. Sofosbuvir plus daclatasvir with or without ribavirin in 551 patients with hepatitis C-related cirrhosis, genotype 4. Aliment Pharmacol Ther. 2018;47:674–679. [PubMed: 29314146](Among 551 Egyptian adults with chronic hepatitis C, genotype 4, with cirrhosis treated with sofosbuvir and daclatasvir with ribavirin for 12 weeks and without ribavirin for 24 weeks, the SVR rate was 92% and serious adverse events arose mostly in those with decompensated cirrhosis, which included hepatic encephalopathy and liver cancer).
- Kwo P, Fried MW, Reddy KR, Soldevila-Pico C, Khemichian S, Darling J, Zamor PJ, et al. Daclatasvir and sofosbuvir treatment of decompensated liver disease or post-liver transplant hepatitis C virus recurrence in patients with advanced liver disease/cirrhosis in a real-world cohort. Hepatol Commun. 2018;2:354–363. [PMC free article: PMC5880197] [PubMed: 29619415](Among 77 patients with chronic hepatitis C with decompensated cirrhosis [n=14] or recurrent hepatitis C after liver transplant treated with sofosbuvir and daclatasvir with or without ribavirin for 24 weeks, the SVR rate was 62% for those with decompensated cirrhosis and 90% with recurrence after transplantation; adverse events were reported in 90% of patients which were serious in 39%, leading to discontinuation of therapy in 13% and resulting in death in 6 [4 were liver related]).
- Sulkowski MS, Feld JJ, Lawitz E, Felizarta F, Corregidor AM, Khalid O, Ghalibv R, et al. Efficacy and safety of 6 or 8 weeks of simeprevir, daclatasvir, sofosbuvir for HCV genotype 1 infection. J Viral Hepat. 2018;25:631–639. [PubMed: 29274193](Among 68 adults with chronic hepatitis C, genotype 1, treated with simeprevir, daclatasvir and sofosbuvir for either 6 weeks [mild fibrosis] or 8 weeks [cirrhosis], the SVR rate was 86% in the 6 week and 100% in the 8 week arm, but relapse was frequent [12%]; there were no therapy related serious adverse events or elevations of serum ALT above 5 times ULN).
- Shiha G, Soliman R, ElBasiony M, Hassan AA, Mikhail NNH. Sofosbuvir plus daclatasvir with or without ribavirin for treatment of chronic HCV genotype 4 patients: real-life experience. Hepatol Int. 2018;12:339–347. [PubMed: 29663115](Among 1168 Egyptian adults with chronic hepatitis C, genotype 4, treated with sofosbuvir and daclatasvir with or without ribavirin for 12 or 24 weeks, the SVR rate was 97% and there were no serious adverse events and only one patient discontinued therapy early for side effects).
- Miotto N, Mendes LC, Zanaga LP, Lazarini MSK, Goncales ESL, Pedro MN, Goncales FL Jr, Stucchi RSB, et al. All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: The role of cirrhosis and comorbidities in treatment response. PLoS One. 2018;13:e0199941. [PMC free article: PMC6038991] [PubMed: 29990371](Among 527 Brazilian patients with chronic hepatitis C treated with interferon free regimens [including sofosbuvir, daclatasvir, ribavirin and simeprevir] between 2015 and 2018, the overall SVR rate was 91% and 22 of 272 patients [8%] with cirrhosis had decompensation during therapy).
- Cheng PN, Chiu YC, Chien SC, Chiu HC. Real-world effectiveness and safety of sofosbuvir plus daclatasvir with or without ribavirin for genotype 2 chronic hepatitis C in Taiwan. J Formos Med Assoc. 2019;118:907–913. [PubMed: 30316677](Among 32 Taiwanese adults with chronic hepatitis C, genotype 2, [50% with cirrhosis, decompensated in 19%] treated with sofosbuvir and daclatasvir for 12 weeks, the SVR rate was 100%; 1 patient had a transient ALT elevation above 5 times ULN, and severe adverse events included a death from heart failure in one patient and transient hepatic encephalopathy in another).
- Poordad F, Shiffman ML, Ghesquiere W, Wong A, Huhn GD, Wong F, Ramji A, et al. ALLY-3C study team. Daclatasvir and sofosbuvir with ribavirin for 24 weeks in chronic hepatitis C genotype-3-infected patients with cirrhosis: a Phase III study (ALLY-3C). Antivir Ther. 2019;24:35–44. [PubMed: 30382942](Among 78 patients with chronic hepatitis C, genotype 3, with compensated cirrhosis treated with sofosbuvir and daclatasvir without ribavirin for 24 weeks, the SVR rate was 87% and there were no deaths, but 8 serious adverse events, but none were liver related).
- Pariente A, Arpurt JP, Rémy AJ, Rosa-Hézode I, Causse X, Heluwaert F, Macaigne G, et al. Association nationale des gastroentérologues des hôpitaux (ANGH). Hepatitis C treatment with all-oral direct-acting antivirals: Effectiveness and tolerance in a multicenter, prospective, observational study from French general hospitals (APROVVIE, ANGH). Presse Med. 2019;48(3 Pt 1):e101–e110. [PubMed: 30853287](Among 1123 patients with chronic hepatitis C treated at 24 French general hospitals with various interferon-free antiviral regimens for 12 or 24 weeks the overall response rate was 91% with lower responses among 553 patients with cirrhosis, two of whom developed hepatic decompensation and died).
- Wahid B. Hepatotoxicity and virological breakthrough of HCV following treatment with sofosbuvir, daclatasvir, and ribavirin in patients previously treated for tuberculosis. J Med Virol. 2019;91:2195–2197. [PubMed: 31347729](A 71 year old man with chronic hepatitis C and decompensated cirrhosis being treated with sofosbuvir, daclatasvir and ribavirin developed virologic breakthrough and worsening ascites after 3 months of therapy).
- El Kassas M, Abdeen N, Omran D, Alboraie M, Salaheldin M, Eltabbakh M, Farghaly R, et al. Safety and efficacy of sofosbuvir/ledipasvir and sofosbuvir/daclatasvir in the treatment of hepatitis C in patients with decompensated cirrhosis. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e877-e882. [PubMed: 34560693](Among 145 Egyptian patients with chronic hepatitis C and decompensated cirrhosis treated with sofosbuvir combined with ledipasvir or daclatasvir, the SVR rate was 88% and 11 patients had to discontinue therapy easy because of ALT elevations or worsening decompensation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Sofosbuvir.[LiverTox: Clinical and Researc...]Review Sofosbuvir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge.[J Clin Virol. 2016]Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge.De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, Cottalorda J, Ollier L, Giordanengo V. J Clin Virol. 2016 May; 78:27-30. Epub 2016 Mar 3.
- Review Hepatitis C (HCV) Agents.[LiverTox: Clinical and Researc...]Review Hepatitis C (HCV) Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Zepatier.[LiverTox: Clinical and Researc...]Review Zepatier.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Sofosbuvir-/Daclatasvir-based therapy for chronic HCV and HCV/hepatitis B virus coinfected patients in Egypt.[Trans R Soc Trop Med Hyg. 2020]Sofosbuvir-/Daclatasvir-based therapy for chronic HCV and HCV/hepatitis B virus coinfected patients in Egypt.Nagaty A, Helmy SH, Abd El-Wahab EW. Trans R Soc Trop Med Hyg. 2020 Feb 7; 114(3):200-212.
- Daclatasvir - LiverToxDaclatasvir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...