NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | 3'-Aza-2'-[18F]fluorofolic acid |
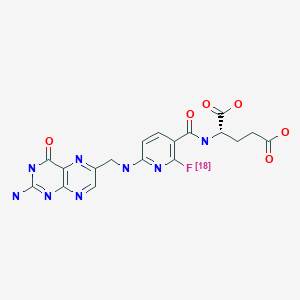
|
| Abbreviated name: | 3'-Aza-2'-[18F]FFA, [18F]6 | |
| Synonym: | ||
| Agent category: | Compound | |
| Target: | Folate receptor | |
| Target category: | Receptor | |
| Method of detection: | Positron emission tomography (PET) | |
| Source of signal: | 18F | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Folic acid is a water-soluble B vitamin (1) that is essential for methylation and DNA synthesis. The primary pathway for entry of folate into cells is through the facilitated transporter, which has a low affinity for folate (Michaelis constant (Km) = 1–5 μM). Epithelial cells in the choroid plexus, kidney, lung, thyroid, spleen, placenta, and thymus also possess a receptor on the cell membrane with higher affinity for folate (dissociation constant (Kd) = 0.5 nM), which allows folate uptake via receptor-mediated endocytosis. Some human epithelial tumor cells have been found to overexpress the folate receptor (2). More than 90% of human ovarian and endometrial cancers express the high-affinity receptor, which is absent in the corresponding normal tissues. Breast, colorectal, renal, and lung carcinomas also overexpress the folate receptor but at lower frequencies (20%–50%). Activated macrophages, but not resting macrophages, have also been found to have the folate receptor (3).
Several folate-based conjugates (111In-DTPA-folate, 99mTc-EC-folate, and 68/67/66Ga-γ-DF-folate) have been studied in tumor imaging (4-7). Bettio et al. (8) reported the synthesis of 18F-labeled folate by reaction of [18F]4-fluorobenzylamine (FBA) with the α- and γ-carboxyl groups of folic acid. [18F]α/γ-FBA-folate has been evaluated as a positron emission tomography (PET) agent for detection of folate receptors in tumors in mice. Ross et al. (9) introduced the 18F radiolabel at the 2' position of the 4-amino-benzoyl moiety in folic acid to form 2'-[18F]fluorofolic acid (2'-[18F]FFA). 2'-[18F]FFA was found to be a specific, high-affinity PET agent for imaging folate receptor-positive tumors in mice. However, the low radiochemical yield (1%–4%) from multistep radiosynthesis limited its routine clinical use. Betzel et al. (10) replaced the phenyl ring in 2'-[18F]FFA with a pyridine ring to produce 3'-Aza-2'-[18F]fluorofolic acid (3'-Aza-2'-[18F]FFA or [18F]6) for use with PET imaging of folate receptor in nude mice bearing human nasopharyngeal carcinoma KB tumors.
Related Resource Links:
- Chapters in MICAD (folate receptor)
- Gene information in NCBI (folate receptor)
- Articles in Online Mendelian Inheritance in Man (OMIM) (folate receptor)
- Clinical trials (folate receptors)
- Drug information in FDA (folate receptors)
Synthesis
[PubMed]
Betzel et al. (10) prepared 3'-Aza-2'-[18F]FFA with a two-step synthesis using a standard nucleophilic radiofluorination ([18F]KF/Kryptofix 2.2.2) reaction of N3-acetyl-3'-aza-2'-chlorofolic acid di-tert-butylester (160ºC, 10 min) and subsequent acid hydrolysis (60ºC, 10 min). Maximal overall radiochemical yield was 9%, with >98% radiochemical purity. The total synthesis time was 110 min, and the specific activity was 35–127 GBq/µmol (0.95–3.43 mCi/µmol) at the end of synthesis. 3'-Aza-2'-[18F]FFA is hydrophilic, with a logD7.4 value of −4.2 ± 0.1. 3'-Aza-2'-[18F]FFA was found to be stable in human blood plasma for 4 h at 37°C, and no defluororination was observed after 1 h of incubation with glutathione and human liver S9-fraction at 37°C.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The human nasopharyngeal carcinoma KB cell line expresses the folate receptor as determined with [3H]folate-binding studies in cultures (8). The mean 50% inhibition (IC50) concentrations for 3'-Aza-2'-FFA, 2'-FFA, and folic acid were 1.4 ± 0.5, 0.9 ± 0.1, and 1.1 ± 0.4 nM (10), respectively. Therefore, 3'-Aza-2'-FFA has a binding affinity to folate receptor comparable to that of native folic acid and 2'-FFA. 3'-Aza-2'-[18F]FFA exhibited ~78% accumulation of added radioactivity, and ~19% was internalized after incubation for 2 h at 37°C with KB cells. Co-incubation with excess folic acid inhibited the radioactivity accumulation by >98%.
Animal Studies
Rodents
[PubMed]
Betzel et al. (10) performed ex vivo biodistribution studies of 5 MBq (0.14 mCi) 3'-Aza-2'-[18F]FFA in nude mice (n = 4/group) bearing KB tumor xenografts at 30, 60, and 90 min after intravenous injection. Tumor accumulation was 11.70 ± 0.87% injected dose/gram (ID/g) at 30 min, 11.86 ± 1.73% ID/g at 60 min, and 12.59 ± 1.77% ID/g at 90 min. The tumor accumulation of 3'-Aza-2'-[18F]FFA was higher than that of [18F]α/γ-FBA-folate (6.65 ± 1.80% ID/g at 125 min) (8) and 2'-[18F]FFA (9.37 ± 1.76% ID/g at 75 min) (9). The organ with the highest maximum accumulation was the kidneys (57.33% ID/g at 90 min), followed by the salivary glands (14.95% ID/g at 60 min), liver (13.72% ID/g at 60 min), gallbladder (9.26% ID/g at 90 min), and stomach (2.83% ID/g at 60 min). The radioactivity levels in the blood and bone at 60 min were 0.55% ID/g and 1.57% ID/g, respectively. Accumulation levels in the brain, lungs, heart, spleen, and intestine were <2% ID/g. No radioactive metabolites were detected in the blood, urine, and tumor at 30 min after injection. The tumor/blood ratios were 11.1, 21.6, and 23.8 at 30, 60, and 90 min, respectively. Pretreatment with folic acid (200 μg/mouse) 10 min before 3'-Aza-2'-[18F]FFA injection reduced radioactivity accumulation at 60 min by 96% in the salivary glands, 85% in the tumors, and 89% in the kidneys. Less reduction was observed in the lungs, heart, spleen, bone, muscle, and stomach. No reduction was observed in the gallbladder, intestine, and liver.
The whole-body distribution of 13 MBq (35 mCi) 3'-Aza-2'-[18F]FFA was also assessed with PET imaging at 120–150 min after injection (10). High accumulation was visualized in the kidneys, salivary glands, gallbladder, urinary bladder, tumors, and liver. The accumulation of radioactivity in the tumors, salivary glands, and kidneys was reduced to near background level after folic acid pretreatment.
References
- 1.
- Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab. 2002;3(2):211–23. [PubMed: 12003352]
- 2.
- Ke C.Y., Mathias C.J., Green M.A. The folate receptor as a molecular target for tumor-selective radionuclide delivery. Nucl Med Biol. 2003;30(8):811–7. [PubMed: 14698784]
- 3.
- Nakashima-Matsushita N., Homma T., Yu S., Matsuda T., Sunahara N., Nakamura T., Tsukano M., Ratnam M., Matsuyama T. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(8):1609–16. [PubMed: 10446858]
- 4.
- Mathias C.J., Hubers D., Low P.S., Green M.A. Synthesis of [(99m)Tc]DTPA-folate and its evaluation as a folate-receptor-targeted radiopharmaceutical. Bioconjug Chem. 2000;11(2):253–7. [PubMed: 10725102]
- 5.
- Mathias C.J., Lewis M.R., Reichert D.E., Laforest R., Sharp T.L., Lewis J.S., Yang Z.F., Waters D.J., Snyder P.W., Low P.S., Welch M.J., Green M.A. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl Med Biol. 2003;30(7):725–31. [PubMed: 14499330]
- 6.
- Mathias C.J., Wang S., Low P.S., Waters D.J., Green M.A. Receptor-mediated targeting of 67Ga-deferoxamine-folate to folate-receptor-positive human KB tumor xenografts. Nucl Med Biol. 1999;26(1):23–5. [PubMed: 10096497]
- 7.
- Mathias C.J., Wang S., Waters D.J., Turek J.J., Low P.S., Green M.A. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J Nucl Med. 1998;39(9):1579–85. [PubMed: 9744347]
- 8.
- Bettio A., Honer M., Muller C., Bruhlmeier M., Muller U., Schibli R., Groehn V., Schubiger A.P., Ametamey S.M. Synthesis and Preclinical Evaluation of a Folic Acid Derivative Labeled with 18F for PET Imaging of Folate Receptor-Positive Tumors. J Nucl Med. 2006;47(7):1153–1160. [PubMed: 16818950]
- 9.
- Ross T.L., Honer M., Muller C., Groehn V., Schibli R., Ametamey S.M. A new 18F-labeled folic acid derivative with improved properties for the PET imaging of folate receptor-positive tumors. J Nucl Med. 2010;51(11):1756–62. [PubMed: 20956469]
- 10.
- Betzel T., Muller C., Groehn V., Muller A., Reber J., Fischer C.R., Kramer S.D., Schibli R., Ametamey S.M. Radiosynthesis and Preclinical Evaluation of 3'-Aza-2'-[(18)F]fluorofolic Acid: A Novel PET Radiotracer for Folate Receptor Targeting. Bioconjug Chem. 2013;24(2):205–14. [PubMed: 23273015]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 2’-[(18)F]Fluorofolic acid.[Molecular Imaging and Contrast...]Review 2’-[(18)F]Fluorofolic acid.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(18)F]α/γ-Fluorobenzylamine-folate.[Molecular Imaging and Contrast...]Review [(18)F]α/γ-Fluorobenzylamine-folate.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (68)Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-1,2-diaminoethane-γ-folate (P3246).[Molecular Imaging and Contrast...]Review (68)Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-1,2-diaminoethane-γ-folate (P3246).Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (68)Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-1,2-diaminoethane-γ-5,8-dideazfolic acid (P3238).[Molecular Imaging and Contrast...]Review (68)Ga-1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid-1,2-diaminoethane-γ-5,8-dideazfolic acid (P3238).Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (66/67/68)Ga-γ-Deferoxamine-folate.[Molecular Imaging and Contrast...]Review (66/67/68)Ga-γ-Deferoxamine-folate.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- 3'-Aza-2'-[18F]fluorofolic acid - Molecular Imaging and Contrast Agent Database ...3'-Aza-2'-[18F]fluorofolic acid - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro