NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | Hyperpolarized sodium 1-[13C]pyruvate |
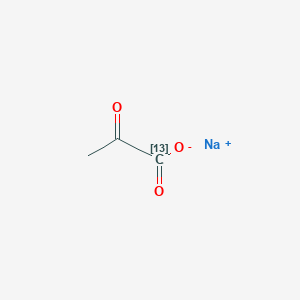
|
| Abbreviated name: | HP[13C]Pyr | |
| Synonym: | Sodium pyruvate-1-13C, Pyruvic acid-1-13C sodium | |
| Agent category: | Small molecule | |
| Target: | Other | |
| Target category: | Other -metabolism | |
| Method of detection: | Magnetic resonance spectroscopic imaging (MRSI), magnetic resonance imaging (MRI) | |
| Source of signal/contrast: | 13C | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Pyruvate (Pyr) is an endogenous substrate enriched at a crossroad of the major energy-generating metabolic pathways in mammalian cells (1). Under most physiological conditions, the bulk of intracellular Pyr is generated endogenously by glycolysis of glucose or oxidation of lactate (Lac) (2). Pyr is then converted into several products through different metabolic pathways in mitochondria or cytoplasm (2, 3). In mitochondria, one of the major pathways is the irreversible transformation of Pyr into acetyl-coenzyme A (acetyl-CoA) and CO2 by pyruvate dehydrogenase (PDH) in the presence of coenzyme nicotinamide adenine dinucleotide (NAD+/NADH). Another major pathway is the conversion of Pyr into oxaloacetate by PDH. Both acetyl-CoA and oxaloacetate are substrates of the tricarboxylic acid cycle (TCA), also known as the Krebs cycle, which generates adenosine triphosphate (ATP) and provides energy to the cell. In the cytoplasm, Pyr is converted into alanine (Ala) by glutamine-Pyr transaminase (GPT) and reversibly oxidized back to Lac by lactate dehydrogenase (LDH). With its ability to increase cardiac mechanical performance and energy reserves, Pyr has been an important therapeutic agent in the treatment of diseases such as dysfunctional myocardium (2). Pyr is also a diagnostic marker in cancers because it is abundantly converted to Lac through anaerobic glycolysis (1). The abnormality of Pyr metabolism in diseased tissue can be detected through quantification of its metabolites. 13C Magnetic resonance spectroscopic imaging (MRSI) is suitable for in vivo quantification because of the inherent chemical shift dispersion of the major metabolites of Pyr (4). The chemical shift value is 178 ppm for Ala, 185 ppm for Lac, 124.5 ppm for CO2, and 173 ppm for Pyr itself (4). Since 13C has low natural abundance (1.1%) and a low gyromagnetic ratio (one quarter compared to proton spin), direct measurement of these metabolites is extremely difficult even with high magnetic fields. The recently developed hyperpolarization technique can increase the signal/noise ratio (SNR) by 10,000-fold (5), allowing direct detection of these metabolites via 13C MRSI.
The signal of nuclear magnetic resonance (NMR) imaging is proportional to the thermal equilibrium polarization of nuclear spins. As a function of magnetic field strength and temperature, the thermal equilibrium polarization normally is very low (i.e., 1×10-6 for 13C at 1.5 T and at body temperature). The hyperpolarization technique increases the polarization of spins by creating an artificial, non-equilibrium distribution of nuclei. Dynamic nuclear polarization (DNP) is an effective technique to hyperpolarize the 13C nuclei of Pyr. Electron spins have a large magnetic moment that is ~660 times stronger than the magnetic moment of proton spins. This strong magnetic moment can be transferred to proton spins through the DNP technique. Theoretically, the DNP enhancement factor for NMR signals depends on the ratio of the electronic gyromagnetic ratio to the nuclear gyromagnetic ratio (γe/γn) in high magnetic fields, which corresponds to a DNP enhancement factor of ~660 for 1H nuclei and ~2600 for 13C nuclei (6). There are four mechanisms accounted for in the DNP process: the Overhauser effect, the solid effect, thermal mixing, and the cross effect or electronuclear cross-polarization (7). The DNP experiments are generally conducted at low temperatures to attenuate competing spin-lattice relaxation processes and avoid loss of efficiency during the polarization transfer (9). Radicals containing unpaired electrons, which have a thermal equilibrium polarization of almost unity when exposed to a magnetic field of ~3 T at a temperature of ~1 K, are added (8). Microwave irradiation near the electron resonance frequency of the radical is used to transfer the polarization from the unpaired electrons on the radicals to the 13C nuclei on substrates. As a result of the short T1 of the electrons (~1 μs), the electrons rapidly regain their polarization. This continuous pumping process can increase the nuclear polarization in the solid material by 20–40% (8). By rapid dissolution, the solid is transformed into an injectable liquid with small polarization losses. The use of DNP produces an enhancement factor of 105 in 13C polarization compared to the thermal equilibrium state at typical magnetic field strengths (8), allowing for direct imaging of metabolites at mM in vivo.
A major difference between the signal of hyperpolarized 13C and the signal of conventional NMR is that the magnetization of hyperpolarized 13C is created outside the magnetic field in a polarizer system (9). Once the hyperpolarization has been created, the polarization will strive to return to the thermal equilibrium level at a rate governed by T1 relaxation time, which typically ranges from a few seconds to several minutes for 13C. The corresponding time window for imaging is approximately a few minutes. Because the 1.1% natural abundance of 13C produces a negligible 13C signal, there is virtually no background signal other than noise from the patient and the coil/receiver system. The injected hyperpolarized 13C-labeled Pyr (HP[13C]Pyr) generates a 13C signal that is linearly proportional to its concentration. In this respect, hyperpolarized 13C MRI behaves in a manner similar to modalities such as positron emission tomography (PET) and single-photon emission tomography (SPECT). Neither PET nor SPECT can distinguish between metabolites and substrates. However, the large chemical shift dispersion in 13C MRSI can be used for quantification of each metabolite (10). The measurement of spatial distribution of metabolites via 13C MRSI allows the establishment of enzyme activity maps (11).
Synthesis
[PubMed]
The preparation of HP[13C]Pyr was described by several authors (10, 12). Two starting materials, [1-13C]Pyr and Tris{8-carboxyl-2,2,6,6-tetra[2-(1-hydroxyethyl)]-benzo(1,2-d:4,5-d’)bis(1,3)dithiole-4-yl}methyl sodium (trityl radical), were commercially available. Both chemicals were dissolved in an aqueous solvent and loaded into a chamber where the sample was cooled to 1.2 K at a pressure of 0.8 mbar. The sample was then irradiated with microwaves (~93.9 GHz) in a helium bath at a magnetic field of 3.35 T for >1 h. After the polarization, the sample was dissolved with hot solvents to produce an injectable solution at approximately 37°C. The level of polarization was estimated by measuring the free induction decays of an aliquot in a NMR spectrometer. The typical polarization was 20–30%.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The evaluation of HP[13C]Pyr influx in EL-4 mouse lymphoma cells was conducted with a 9.4-T imager (11). After adding HP[13C]Pyr into the cells, the conversion of Pyr to Lac produced an increased Lac carboxyl signal that decayed at an apparent T1 of 40 s. The 13C peak intensities for Pyr and Lac were fitted to a modified two-site exchange Bloch equation to extract the apparent exchange rate constants. These rate constants were used to evaluate the LDH activity and Pyr influx during drug treatment. Etoposide was an inhibitor of the enzyme topoisomerase II used in chemotherapy for various malignant carcinomas. A 16-h treatment of the tumor cells with etoposide caused ~30–40% apoptosis and 10–15% necrosis in the cells. This induced ~80% reduction in Pyr flux, which was decreased from 54 ± 15 nmol/s per 108 cells to 9.0 ± 1.5 nmol/s per 108 cells. The LDH activity was nearly the same: 5.43 ± 0.34 units/107 cells in control cells and 5.37 ± 0.27 units/107 cells in drug-treated cells. A 20-h treatment with etoposide produced a 45% necrosis in the cells. The LDH activity also decreased significantly to 2.53 ± 0.32 units/107 cells. The levels of necrosis and apoptosis were further confirmed by flow cytometry.
The metabolism of HP[13C]Pyr in isolated rat hearts was examined at 14.1 T (10). Hearts from adult rats were rapidly excised and perfused at 37°C using standard Langendorff methods. After each aorta was cannulated, each heart was placed in a NMR tube that was then filled with recirculated medium containing fatty acids and 2 mM HP[13C]Pyr with 20–30% polarization. Fatty acids with even or odd carbons were used to modulate the influx of PDH. Propionate (a three-carbon fatty acid) activated 90% PDH and stimulated PDH flux, whereas even-carbon fatty acid strongly inhibited PDH. Experiments were performed under ideal conditions for detected downstream metabolites such as H13CO3- (160.9 ppm) and 13CO2 (124.5 ppm) in the presence of HP[13C]Pyr alone as well as in the presence of HP[13C]Pyr with even or odd fatty acids. The intensity of the H13CO3- and 13CO2 resonances in the hearts reached a maximum of ~20 s after infusion of HP[13C]Pyr. The ratio of the H13CO3- resonance area to the 13CO2 area was 7.03 ± 0.5 and remained constant until T1 relaxation began to destroy the 13CO2 signal. In the presence of octanoate, a medium chain fatty acid, H13CO3- and 13CO2 were nearly invisible and did not recover even in the presence of propionate. This result suggested an effective competition of octanoate with Pyr for supplying acetyl-CoA to the heart.
Animal Studies
Rodents
[PubMed]
The dynamic spectra of HP[13C]Pyr were collected in rats at 3 T (4). Healthy rats were injected intravenously with 3 ml of HP[13C]Pyr solution at 79 mM in 12 s. Chemical shift imaging (CSI) of a thick slab was initiated ~30 s after the injection of HP[13C]Pyr with a dual-tuned volume coil was started, and the CSI were acquired every 3 s. The produced images for a 90-mm slab reflected a combined effect of metabolism, blood flow, and T1 relaxation. The results demonstrated that the conversion of Pyr to its metabolites, including Lac, Ala, and bicarbonate, occurred within a minute of injection. Among these metabolites, Ala was observed primarily in skeletal muscle and liver; Lac and bicarbonate were relatively concentrated in the vasculature and kidneys.
The metabolism of HP[13C]Pyr in lymphoma tumors was examined in mice at 9.4 T (11). After intravenous injection of 0.2 ml of HP[13C]Pyr solution at 75 mM within 3 s, 13C magnetic resonance spectroscopy (MRS) and 13C MRSI were used to evaluate the flux between Pyr and Lac in the tumors. A series of images was acquired to calculate an enzyme activity map. The experiments were performed in mice with and without etoposide treatment. There was a substantial signal from Pyr but only a small signal from Lac in drug-treated and untreated mice. The signal intensities were fitted to a two-site exchange Bloch equation to extract apparent exchange rate constants and spin-lattice relaxation times. A treatment with etoposide resulted in 37 ± 4% death of tumors versus 5 ± 1% in the control group. This led to ~25% reduction in the rate constant of Pyr, which decreased from 0.075 ± 0.011 s-1 to 0.056 ± 0.005 s-1. The decreased flux between Pyr and Lac were demonstrated in a plot in terms of the labeled Lac/Pyr ratio. Pyr concentration in excised tumors was 0.55 ± 0.19 μmol/g in control tumors and 0.75 ± 0.48 μmol/g in drug-treated tumors as determined by 1H NMR.
The metabolism of HP[13C]Pyr was also examined in P22 tumors implanted in six rats (1). 13C-Labeled CSI images were collected for a slice 10 mm thick with a 5×5-mm2 in-plane pixel size at 1.5 T. HP[13C]Pyr was infused in 14 s at a dose of 0.79 mmol/kg. The 13C CSI scans were acquired starting 30 s after the infusion began. All tumors showed significantly higher Lac content within 30 s than the normal tissue. The metabolic conversion of Pyr into Ala and Lac was found in all rats, but the Lac production was especially pronounced in all the tumors. HP[13C]Pyr was used to evaluate the Lac production in transgenic adenocarcinomas of mouse prostate (TRAMP) mice at 3 T (12). 13C MRI or 13C MRSI were collected for both primary and metastatic tumors. The primary tumors and lymph node metastases showed much higher Lac levels compared to the normal mouse prostates.
Human Studies
[PubMed]
No publication is currently available.
NIH Support
RR 02584, HL 34557, EB005363, EB007588
References
- 1.
- Golman K. , Zandt R.I. , Lerche M. , Pehrson R. , Ardenkjaer-Larsen J.H. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006; 66 (22):10855–60. [PubMed: 17108122]
- 2.
- Mallet R.T. Pyruvate: metabolic protector of cardiac performance. Proc Soc Exp Biol Med. 2000; 223 (2):136–48. [PubMed: 10654616]
- 3.
- Koukourakis M.I. , Giatromanolaki A. , Sivridis E. , Gatter K.C. , Harris A.L. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 2005; 7 (1):1–6. [PMC free article: PMC1490315] [PubMed: 15736311]
- 4.
- Kohler S.J. , Yen Y. , Wolber J. , Chen A.P. , Albers M.J. , Bok R. , Zhang V. , Tropp J. , Nelson S. , Vigneron D.B. , Kurhanewicz J. , Hurd R.E. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med. 2007; 58 (1):65–9. [PubMed: 17659629]
- 5.
- Ardenkjaer-Larsen J.H. , Fridlund B. , Gram A. , Hansson G. , Hansson L. , Lerche M.H. , Servin R. , Thaning M. , Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003; 100 (18):10158–63. [PMC free article: PMC193532] [PubMed: 12930897]
- 6.
- Hall D.A. , Maus D.C. , Gerfen G.J. , Inati S.J. , Becerra L.R. , Dahlquist F.W. , Griffin R.G. Polarization-enhanced NMR spectroscopy of biomolecules in frozen solution. Science. 1997; 276 (5314):930–2. [PubMed: 9139651]
- 7.
- Joo C.G. , Hu K.N. , Bryant J.A. , Griffin R.G. In situ temperature jump high-frequency dynamic nuclear polarization experiments: enhanced sensitivity in liquid-state NMR spectroscopy. J Am Chem Soc. 2006; 128 (29):9428–32. [PubMed: 16848479]
- 8.
- Mansson S. , Johansson E. , Magnusson P. , Chai C.M. , Hansson G. , Petersson J.S. , Stahlberg F. , Golman K. 13C imaging-a new diagnostic platform. Eur Radiol. 2006; 16 (1):57–67. [PubMed: 16402256]
- 9.
- Olsson L.E. , Chai C.M. , Axelsson O. , Karlsson M. , Golman K. , Petersson J.S. MR coronary angiography in pigs with intraarterial injections of a hyperpolarized 13C substance. Magn Reson Med. 2006; 55 (4):731–7. [PubMed: 16538605]
- 10.
- Merritt M.E. , Harrison C. , Storey C. , Jeffrey F.M. , Sherry A.D. , Malloy C.R. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007; 104 (50):19773–7. [PMC free article: PMC2148374] [PubMed: 18056642]
- 11.
- Day S.E. , Kettunen M.I. , Gallagher F.A. , Hu D.E. , Lerche M. , Wolber J. , Golman K. , Ardenkjaer-Larsen J.H. , Brindle K.M. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007; 13 (11):1382–7. [PubMed: 17965722]
- 12.
- Chen A.P. , Albers M.J. , Cunningham C.H. , Kohler S.J. , Yen Y.F. , Hurd R.E. , Tropp J. , Bok R. , Pauly J.M. , Nelson S.J. , Kurhanewicz J. , Vigneron D.B. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn Reson Med. 2007; 58 (6):1099–106. [PubMed: 17969006]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Hyperpolarized [(13)C]-2-hydroxyethylpropionate.[Molecular Imaging and Contrast...]Review Hyperpolarized [(13)C]-2-hydroxyethylpropionate.Zhang H. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Hyperpolarized α-keto[1-(13)C]isocaproate as a (13)C magnetic resonance spectroscopic agent for profiling branched chain amino acid metabolism in tumors.[Molecular Imaging and Contrast...]Review Hyperpolarized α-keto[1-(13)C]isocaproate as a (13)C magnetic resonance spectroscopic agent for profiling branched chain amino acid metabolism in tumors.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Hyperpolarized [1,4-(13)C(2)]fumarate as an imaging agent of tumor cell death in vivo.[Molecular Imaging and Contrast...]Review Hyperpolarized [1,4-(13)C(2)]fumarate as an imaging agent of tumor cell death in vivo.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Hyperpolarized (13)C-labeled bicarbonate (H(13)CO(3)(-)) for in vivo pH measurement with (13)C magnetic resonance spectroscopy.[Molecular Imaging and Contrast...]Review Hyperpolarized (13)C-labeled bicarbonate (H(13)CO(3)(-)) for in vivo pH measurement with (13)C magnetic resonance spectroscopy.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (15)N-Labeled 4-oxo-2,2,6,6-tetramethyl-piperidine-1-oxyl.[Molecular Imaging and Contrast...]Review (15)N-Labeled 4-oxo-2,2,6,6-tetramethyl-piperidine-1-oxyl.Zhang H. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Hyperpolarized sodium 1-[13C]pyruvate - Molecular Imaging and Contrast Agent Dat...Hyperpolarized sodium 1-[13C]pyruvate - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro