The nutritional demands imposed by breastfeeding depend primarily on the absolute quantities of nutrients transferred from the mother to the infant through the milk. Thus, in considering recommendations for maternal nutrition during lactation, it is essential to carefully examine both the volume and composition of human milk. Milk volume is the focus of this chapter; Chapter 6 covers composition.
The subcommittee addressed the following questions in its review of milk volume:
- Is the volume or energy content of human milk compromised when intake of energy or other nutrients is restricted during lactation? Do maternal body fat or other nutrient stores modify this relationship?
- Does energy supplementation or increased intake of protein or fluid increase milk volume?
- What other factors must be considered when examining the effects of maternal nutrition on milk volume?
These questions can be examined only in the context of a clear understanding of the regulation of milk production in humans. For this reason, this chapter includes consideration of the physiologic control of lactation and of the infant's role in this process, in addition to maternal factors such as age, parity, stress, substance use, and nutrition.
Measurement Of Milk Volume
A key element defining lactation performance is the total amount of milk produced. The amount of milk transferred to the infant affects the infant's nutrient intake and the mother's nutrient requirements. In this report, the subcommittee distinguishes between milk intake by the infant (also referred to as milk volume) and milk production by the mother. Ordinarily, production is measured as intake, but it may exceed intake if extra milk is removed from the breast and is not consumed by the infant or the infant regurgitates milk.
The most widely accepted method for measuring milk intake is test weighing, a procedure in which the infant is weighed before and after each feeding, preferably using a balance scale accurate to ±1 g. In this method, milk intake is usually underestimated by approximately 1 to 5% (Brown et al., 1982; Woolridge et al., 1985) because of evaporative water loss from the infant between weighings. The procedure is potentially disruptive to the nursing patterns of the mother and infant, especially if nursing is very frequent or the infant nurses occasionally during the night while sleeping with the mother. Under conditions typical of breastfeeding mothers in the United States, the method is generally well accepted (Dewey and Heinig, 1987). Intake is usually reported in grams because they are the unit of measurement used in test weighing; the density of human milk is approximately 1.03 g/ml (Neville et al., 1988; Woolridge et al., 1985). Newer techniques for measuring breast milk intake based on the use of stable isotopes have been developed, but few data obtained with them have been published (Butte et al., 1988; Coward et al., 1982; Fjeld et al., 1988; Wong et al., 1990). Maternal milk production can be measured mechanically by extracting all the milk or by using a combination of test weighing and extraction of residual milk.
Normal Range Of Milk Intake And Production
There is a very wide range in milk intake among healthy, exclusively breastfed infants. Figure 5-1 illustrates variability in infant milk intake during established lactation. In industrialized countries, milk intakes average approximately 750 to 800 g/day in the first 4 to 5 months, but range from approximately 450 to 1,200 g/day (Butte et al., 1984b; Chandra, 1981; Dewey and Lönnerdal, 1983; Hofvander et al., 1982; Lönnerdal et al., 1976; Neville et al., 1988; Pao et al., 1980; Picciano et al., 1981; Rattigan et al., 1981; Wallgren, 1944/1945; Whitehead and Paul, 1981). Recent data from developing countries indicate a similar mean level of intake when a rigorous methodology for measuring milk volume is used (Brown et al., 1986b; Prentice et al., 1986) (see Figure 5-2).
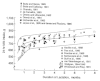
FIGURE 5-1
Milk intakes during established lactation from studies meeting defined criteria, from Neville et al. (1988) with permission. The lines show the smoothed mean ± standard deviation, from Neville et al. (1988). The points represent average intakes (more...)

FIGURE 5-2
Average intake of human milk by infants at age 3 months in industrialized and developing countries. Data were compiled from studies later than the year 1975. Adapted from Prentice et al. (1986) with permission.
Milk intake after the first 4 to 5 months varies even more widely. In U.S. infants who were breastfed for at least 12 months and were given solid foods beginning at 4 to 7 months, milk intake averaged 769 g/day (range, 335 to 1,144 g/day) at 6 months (N = 56), 637 g/day (range, 205 to 1,185 g/day) at 9 months (N = 46), and 445 g/day (range, 27 to 1,154 g/day) at 12 months (N = 40) (Dewey et al., in press).
Several studies indicate that potential milk production in humans is considerably higher than the average intake by single infants. Kaucher and colleagues (1945) measured maximum milk output with intrusive and tedious mechanical methods to extract all the mother's milk and reported that production averaged almost 1,200 g/day at 6 to 10 days post partum. This level is much higher than the 500 to 700 g/day consumed by breastfed infants at the same age (Casey et al., 1986; Saint et al., 1984). In two separate studies, milk production increased by 15 to 40% when a breast pump was used to remove additional milk after feedings (Dewey and Lönnerdal, 1986; Neville and Oliva-Rasbach, 1987). Mothers who exclusively breastfeed twins or triplets can produce 2,000 to 3,000 g/day, although this involves nursing an average of 15 or more times per day (Saint et al., 1986). Women who express surplus milk for a milk bank have been shown to produce as much as 3,000 g/day (Macy et al., 1930).
Breast Development And Physiology
The data discussed above illustrate that lactation is a physiologic process with a great deal of plasticity—that is, milk production can be regulated up or down, depending on the degree of stimulation to the mammary gland. The processes leading to a woman's ability to secrete milk start long before lactation commences.
Mammary development begins in early fetal life and extends through puberty; it resumes early in pregnancy. The process is influenced by several hormones, including estrogens, progesterone, and lactogenic hormones (Neville and Neifert, 1983). Mammary gland enlargement is especially pronounced during the first half of pregnancy, when lobuloalveolar growth is accompanied by differentiation of the epithelial cells. Both prolactin and placental lactogen may initiate this enlargement, although either one alone may provide sufficient stimulus for mammary development. Insufficient development before or during pregnancy may contribute to lactation failure (Neifert and Seacat, 1986). The prevalence of this problem has not been studied but is likely to be very low.
Lactogenesis, defined as ''the onset of copious milk secretion around parturition" (Neville and Neifert, 1983, p. 108), is believed to be triggered by the decrease in progesterone following parturition. Incomplete delivery of the placenta has been shown to delay lactogenesis, presumably because it is accompanied by continued high levels of progesterone (Neifert et al., 1981). Prolactin is believed to be essential for normal lactogenesis, but the mechanism or mechanisms for its influence are not clearly understood. Once milk production has begun, the hormonal mechanisms maintaining milk secretion are believed to depend primarily on the actions of prolactin and oxytocin. Prolactin is generally understood to promote milk synthesis and secretion into the alveolar spaces. Its metabolic effects include promotion of fat synthesis in mammary tissue, increased fat mobilization at other body sites, enhancement of casein synthesis and casein messenger RNA formation in the rat and rabbit, and stimulation of milk α-lactalbumin and lactose levels in cows (Horrobin, 1979). Prolactin levels are influenced by the amount and frequency of suckling, but vary considerably among women producing comparable volumes of milk (Martin, 1983; Noel et al., 1974; Strode et al., 1986; Tyson et al., 1978).
Oxytocin is secreted by the maternal pituitary in response to suckling and in turn stimulates contraction of the myoepithelial cells, leading to milk ejection. This milk-ejection reflex, or let-down, moves milk from the storage alveoli to the lacteal sinuses, allowing the milk to be easily removed by the infant (Woolridge and Baum, 1988).
Milk production may also be governed by local negative feedback within each breast, referred to by Wilde et al. (1988) as "autocrine" control. These investigators reported that a constituent of milk whey protein inhibits milk secretion in a dose-dependent manner in goats. As milk builds up in the mammary gland between feedings, the concentration of this inhibitor presumably increases and thus retards and eventually stops milk production. Removal of milk eliminates the inhibitory effect and milk production resumes or increases. This inhibitory mechanism could explain why two breasts of the same woman with different milk removal rates can produce very different quantities of milk.
Breast engorgement and the resulting increased pressure in and distension of the mammary gland also lead to decreased milk production. Studies in animals suggest that when milking ceases, distension of the alveoli caused by pooling of the milk brings about a decrease in milk secretion within 6 hours (Neville and Neifert, 1983).
Infant Factors Influencing Milk Production And Transfer
Management of lactation during the first few weeks is critical to the establishment of an adequate milk supply. Successful lactation depends on several factors, such as proper positioning of the infant at the breast, precautions to avoid sore nipples, frequent feedings, avoidance of formula feeding, and timing of feedings to coincide with the infant's desire to suck. These factors are discussed in detail in breastfeeding guides (e.g., Goldfarb and Tibbetts, 1989, Lawrence, 1989). For the purposes of this report, the subcommittee restricted its discussion to infant characteristics that may influence milk volume, such as birth weight, sucking strength, gestational age at delivery, and illness, and to maternal characteristics, such as age, parity, stress, substance use, and nutritional status. These characteristics appear to be those that are most likely to affect milk volume if they influence the frequency, intensity, or duration of sucking by the infant.
Nursing Frequency
During the early postpartum period, when the milk supply is being established (Lawrence, 1985), there is a positive association between nursing frequency and milk production (de Carvalho et al., 1983, 1985; Hopkinson et al., 1988; Salariya et al., 1978). In a study of 32 mothers of preterm infants, optimal milk production was achieved when milk was pumped five or more times per day during the first month post partum (Hopkinson et al., 1988). Among women breastfeeding full-term infants, mean nursing frequency of 10 ± 3 times per day during the first 2 weeks post partum was associated with adequate milk production (de Carvalho et al., 1982). Although there is considerable interindividual variability in infants' need to suck, nursing on demand (at least eight times per day in the early postpartum period) is recommended to provide the necessary degree of hormonal stimulation to the mammary gland.
Once lactation is established, cross-sectional studies of well-nourished, exclusively breastfeeding women nursing 4 to 16 times per day indicate that there is little, if any, relationship between nursing frequency and infant milk intake (Butte et al., 1984a; de Carvalho et al., 1982; Dewey et al., 1986) or between basal serum prolactin levels and milk volume (Lunn et al., 1984; Noel et al., 1974; Strode et al., 1986; Tyson et al., 1978). These findings do not imply, however, that the milk output of individual mothers cannot be altered by changing nursing frequency. At least one report illustrates that limiting the number of feedings can reduce milk production (Egli et al., 1961), and during gradual weaning, it is obvious that mothers are able to decrease their infant's intake of human milk by nursing less often. Thus, although some infants are capable of consuming adequate amounts of milk by feeding only four to five times a day, women who are concerned about the adequacy of their milk supply are well advised to nurse more often.
Birth Weight
Prentice et al. (1986) and Dewey et al. (1986) observed an association between infant birth weight and volume of milk intake. This appears to be related to the greater sucking strength, frequency, or feeding duration among larger infants—all of which could increase milk volume. Pollitt and colleagues (1978) demonstrated that infant weight at 2 days and at 1 month of age was strongly correlated with sucking strength, which appeared to be responsible for the large variations in intake per feeding among formula-fed infants. Among breastfed infants, de Carvalho et al. (1982) found a positive relationship between infant birth weight and frequency and duration of feeding during the first 14 days post partum.
Gestational Age at Delivery
The interaction of gestational age and birth weight may have a stronger influence on milk intake than does either one alone, because preterm infants (especially those born at <34 weeks of gestation) may be too weak or immature to suck effectively. Studies of the volume of milk produced by mothers of preterm infants are complicated by the fact that many mothers must pump milk for several days or weeks before the infant can suck directly from the breast. The degree of maternal motivation to breastfeed plays a large role in the success of this phase.
Self-Regulation
Self-regulation of milk intake was studied among 18 exclusively breastfed infants of mothers who increased their milk supply by expressing extra milk daily for 2 weeks (Dewey and Lönnerdal, 1986). On average, these infants took in more milk immediately following this 2-week period, but about half of them returned to near baseline levels of milk intake after another 1 to 2 weeks. Net change in milk intake at the end of the study was greater among heavier infants and was not associated with baseline milk volume. This indicates that milk intake was influenced more by infant demand than by maternal capacity for milk production. In a subsequent study, Dewey et al. (in press) showed that residual milk volume (the difference between the amount that can be extracted by pump compared with usual infant intake) averages about 100 g/day, even among mothers whose infants consume relatively low amounts of milk (<650 g/day). Likewise, Woolridge and Baum (1988) demonstrated that when 29 mothers randomly selected the breast from which to feed the baby first, intake from the second breast was only about 60% of the amount taken from the first breast. These results illustrate that infants ordinarily do not take all the available milk and therefore govern their own intake to a considerable extent.
Self-regulation of milk intake by infants was also demonstrated by Stuff and Nichols (1989), who studied 45 breastfed infants before and after they began consuming solid foods. Energy intake per kilogram of body weight of these infants during exclusive breastfeeding was considerably lower than the Recommended Dietary Allowance (NRC, 1989) and did not increase after solid foods were introduced. Instead, the infants responded to solid foods by reducing breast milk intake, thereby maintaining constant levels of energy intake. Similarly, Nommsen and colleagues (1989) found that solid foods displaced energy intake from human milk in 6-month-old infants even though they were breastfed on demand.
Factors influencing the infant's demand for milk have not been studied thoroughly. When the milk supply is ample, the infant's milk intake is positively associated with infant weight. Because the mean weight of boys is heavier than that of girls of the same age, intake is also associated with the sex of the infant. Illness of the infant may reduce appetite and therefore milk intake. In The Gambia, Prentice et al. (1986) observed that decreases in milk intake by infants during the wet season (a period of food scarcity) were usually associated with gastrointestinal or respiratory infections. As described later in this chapter, maternal supplementation did not prevent the seasonal decline in milk volume, indicating that this pattern was probably not due to maternal nutritional limitations but to either altered feeding practices or illness-induced anorexia among the infants. From the Gambian data, it is difficult to separate the influence of these factors. In contrast, Brown et al. (in press) found that milk intake among breastfed infants in Peru remained constant, whereas intake of other foods was reduced during illness.
Maternal Factors
Age and Parity
Maternal variables such as age and parity have little or no relationship to milk production in most populations (as measured by the infant's intake of human milk). There have been few studies of the volume of milk produced by adolescent mothers. In one study, Lipsman et al. (1985) found that milk intake appeared adequate (based on measures of infant growth) for 22 of the 25 infants of well-nourished, lactating teenagers. Among women aged 21 to 37, no association was observed between maternal age and infant milk intake (Butte et al., 1984b; Dewey et al., 1986), despite Hytten's (1954) concerns that milk yield may decrease because of "disuse atrophy" after age 24.
There is some evidence that milk production on the fourth day post partum is higher among multiparous than it is among primiparous women (Zuppa et al., 1988); however, once lactation is established, there is no statistically significant association between parity and infant milk intake in well-nourished populations (Butte et al., 1984a; Dewey et al., 1986; Rattigan et al., 1981). In The Gambia, infants of mothers who had borne 10 or more children had low milk intakes (Prentice, 1986), but this level of parity is rarely seen in industrialized countries.
Stress and Acute Illness
Maternal anxiety and stress, which may be exacerbated by poor lactation management, are believed to influence milk production by inhibiting the milk-ejection reflex. This reflex usually operates well in women who are relaxed and confident of their ability to breastfeed. In tense women, however, the reflex may be impaired. Limited documentation of the effects of stress or relaxation on let-down is provided by Newton and Newton (1948, 1950) and Feher et al. (1989), but further studies are needed to explore the effects of various types of maternal stress, especially chronic anxiety and tension, on milk production. There are also few data concerning the potential influence of common short-term maternal illnesses on breastfeeding. It is known, however, that mothers can and should continue to nurse when they have mastitis (Lawrence, 1989).
Substance Use
Maternal behavior such as cigarette smoking and alcohol consumption may influence both milk production and milk composition. Potential consequences to the infant are discussed in Chapter 7; this section is restricted to effects on milk volume.
Cigarette Smoking
Smoking may reduce milk volume through an inhibitory effect on prolactin or oxytocin levels. Studies in rats have shown decreased release of prolactin in response to suckling and decreases in both milk output and pup growth upon exposure to nicotine or tobacco fumes (Blake and Sawyer, 1972; Ferry et al., 1974; Hamosh et al., 1979; Terkel et al., 1973). Smoking is also known to stimulate release of adrenaline, which in turn can inhibit oxytocin release (Cross, 1955). Studies in humans show a consistent association between smoking and early weaning (Lyon, 1983; Matheson and Rivrud, 1989; Whichelow and King, 1979), but milk volume was not measured directly in those studies. Since smoking is usually more common among women of lower socioeconomic status and educational level than among more advantaged women, it is possible that the smoking itself is not the factor that contributes to early weaning. However, both Lyon (1983) and Matheson and Rivrud (1989) reported a lower prevalence of breastfeeding at 6 to 12 weeks post partum among smokers compared with nonsmokers even within the same socioeconomic group. Furthermore, Matheson and Rivrud (1989) found a greater incidence of colic among infants of breastfeeding mothers who smoked.
Andersen and coworkers (1982) demonstrated that women who smoked 15 or more cigarettes per day had 30 to 50% lower basal prolactin levels on days 1 and 21 post partum than did nonsmokers, although the suckling-induced rise in prolactin was not different between groups. Oxytocin levels were not influenced by smoking. Since the infants of smokers tend to have average birth weights that are approximately 200 g lower than those of the infants of nonsmokers (IOM, 1990) (which is the case in the study by Andersen et al. [1982]), and since lower birth weight may decrease infant demand for milk and thus both prolactin levels and milk volume, it is difficult to separate cause and effect in these studies. Nonetheless, the evidence from investigations in both animals and humans strongly suggests that smoking has an adverse effect on milk volume.
Alcohol Consumption
The influence of alcohol consumption on milk production is less straightforward than that of smoking. It has long been maintained that small amounts of alcoholic beverages can help breastfeeding mothers to relax and thus foster effective functioning of the milk-ejection reflex (Lawrence, 1989). On the other hand, ethanol is a known inhibitor of oxytocin release (Fuchs and Wagner, 1963).
Two studies have demonstrated that the milk-ejection reflex can be at least partially blocked by maternal alcohol intake and that this effect is dose dependent (Cobo, 1973; Wagner and Fuchs, 1968). Wagner and Fuchs (1968) measured uterine contractions during suckling as an indicator of oxytocin release. At ethanol doses of 0.5 to 0.8 g/kg of maternal body weight, uterine activity was 62% of normal; at 0.9 to 1.1 g/kg, it was 32% of normal. Cobo (1973) measured the milk-ejection reflex by recording intraductal pressure in the mammary gland. He observed no effect of ethanol intake at doses below 0.5 g/kg; but the milk-ejection response was inhibited by 18.2, 63.2, and 80.4% at doses of 0.5 to 0.99, 1.0 to 1.49, and 1.5 to 1.99 g/kg, respectively. At 0.5 to 0.99 g/kg, this effect was not statistically significant, but at 1.0 to 1.49 g/kg, the milk-ejection reflex was completely blocked in 6 of the 14 subjects. The effect of alcohol on this reflex was not apparent when oxytocin was injected, indicating that the inhibition involved the release rather than the activity of oxytocin.
For an average woman weighing 60 kg (132 lb), an ethanol dose of 0.5 g/kg of body weight corresponds to approximately 2 to 2.5 oz of liquor, 8 oz of wine, or 2 cans of beer. Thus, these studies indicate that the adverse effects of alcohol consumption on the milk-ejection reflex are apparent only at relatively high intakes.
Oral Contraceptive Agents
The impact of oral contraceptive agents on lactation performance has been the subject of numerous studies (see reviews by Koetsawang [1987] and Lönnerdal [1986]). In the United States, 12.6% of lactating women who participated in the 1982 National Survey of Family Growth reported that they used oral contraceptives; this proportion was much higher among blacks (26.9%) than among whites (11.7%) (Ford and Labbok, 1987).
In providing guidance to women planning to use oral contraceptives, it is important to consider the composition and dosage of the pill and the intended duration of exclusive breastfeeding. In most studies conducted on the subject, the use of combined estrogen and progestin pills has been associated with reduced milk volume and duration of breastfeeding (Koetsawang, 1987; Lönnerdal, 1986). A recent multi-center, randomized double-blind trial in Hungary and Thailand demonstrated that even low-dose combined oral contraceptives (150 µg of levonorgestrel and 30 µg of ethinyl estradiol) have this effect: between 6 and 24 weeks post partum, the rate of milk volume decrease in women taking these pills was about twice the rate observed in control women (WHO Task Force on Oral Contraceptives, 1988). The nitrogen content of milk also was lower in those taking the combined pills, but there was no consistent effect on lactose or fat concentrations.
In contrast, no effect on milk volume or composition has been associated with progestin-only pills (Koestsawang, 1987; Lönnerdal, 1986; WHO Task Force on Oral Contraceptives, 1988). Although progesterone is known to inhibit lactogenesis, once lactation has been established it has no known inhibitory effect on milk production, possibly because progesterone binding sites are apparently not present in lactating tissues (Neville and Neifert, 1983). Further, there are substantial chemical differences between natural progesterone and synthetic progestins. Progestin-only pills have been found to be slightly less effective contraceptives than combined pills in studies of nonlactating women (Winikoff et al., 1988), but it is not known if this difference in effectiveness applies to lactating women as well. Progestin-only pills are also associated with altered menstrual cycles in nonlactating women, but the prevalence of this dysfunction is unknown in lactating women, who are likely to have a longer period of postpartum amenorrhea. For lactating women who wish to use oral contraceptives and maintain milk production, the World Health Organization states that progestin-only pills are the preferred choice (WHO Task Force on Oral Contraceptives, 1988).
Maternal Nutrition and Energy Balance
This section begins with consideration of maternal energy balance during lactation; this is followed by discussions of protein and fluid intakes. Studies on the influence of other nutrients have dealt primarily with milk composition, rather than volume, and are discussed in Chapter 6.
In its review, the subcommittee gave greatest weight to evidence with the greatest relevance to making causal inferences to human populations. Causal relationships can be most definitively demonstrated in intervention studies with randomized designs; however, very few studies of the effects of maternal nutrition on milk volume meet this criterion. Thus, the subcommittee also reviewed observational studies in humans, which are useful in establishing associations between factors, and studies in animals, which can suggest hypotheses to be tested in humans. The following sections begin with discussions of data on animals and progress to studies in humans, reflecting the chain in which evidence is usually accumulated.
In examining the evidence relating energy balance to milk volume, the subcommittee addressed three major questions:
- Is the volume of human milk affected if energy intake is curbed or supplemented during lactation?
- Do maternal fat stores or weight relative to height affect the relationship between energy deficit and milk volume?
- Are the mechanisms of energy utilization during lactation relevant to the volume of milk produced by lactating women?
Energy Restriction and Milk Volume
Several investigators have developed animal models of malnutrition during lactation, primarily in rats. Studies by Warman and Rasmussen (1983), Young and Rasmussen (1985), and Kliewer and Rasmussen (1987) illustrate that milk yield is decreased by dietary restriction and that the decrease is more pronounced in rats restricted before and during lactation than it is in those restricted only during lactation. Milk yield was reduced only 12.5% in dams fed 75% of ad libitum intake by controls, suggesting that the underfed animals compensated for dietary restriction in some way. In contrast, there was a dramatic (52%) decrease in milk yield in rats restricted to 50% of ad libitum intake (Young and Rasmussen, 1985). These results suggest that there may be a threshold below which lactation can no longer be protected when food intake is restricted. Similar findings have been reported by Roberts et al. (1985) in studies of baboons. Among animals restricted to 80% of ad libitum intake, milk output was not significantly reduced, whereas milk output decreased 20% in those restricted to 60% of ad libitum intake. Reduced physical activity may protect milk output at moderate levels of energy restriction but not at high levels, when body stores were shown to be mobilized at a rapid rate in the baboons. However, this possible effect of physical activity has not been studied.
The relative energy costs of lactation are much lower for humans than for most other species, and it is not known whether there is an energy threshold for humans. Prentice and Prentice (1988) report that energy costs at peak milk output, as a function of maternal body weight, are 4- to 15-fold lower for humans than for either laboratory or domesticated animals. For example, the energy requirements of lactation in humans can be met by increasing energy intake by approximately 25%, whereas in rats, energy intake must increase by 300% or more. Thus, a similar reduction in energy intake as a percentage of total intake is likely to result in a smaller decrease in milk volume of humans and other primates than of litter-bearing animals.
Observational studies of the relationship between maternal energy intake and milk volume in human populations have yielded mixed results. Despite much lower reported energy intakes among women in developing countries compared with their counterparts in industrialized countries, average milk volumes of both groups at 3 months post partum are similar (Prentice et al., 1986, see Figure 5-2). In industrialized countries, Strode et al. (1986) found no association between maternal energy intake and infant milk intake, whereas Butte et al. (1984a) and Prentice et al. (1986) reported a weak correlation in early lactation. Such an association may reflect reverse causation: women who produce more milk might consume more food because of greater appetite. (In rats, food intake is stimulated by lactation [Roberts and Coward, 1985] or by experimentally manipulating levels of serum prolactin [Moore et al., 1986].) In The Gambia, Prentice and colleagues (1986) found a striking association between seasonal patterns of maternal energy intake and infant milk intake but concluded that this association reflected changes in breastfeeding patterns and infant illnesses rather than maternal undernutrition.
There have been several attempts to document the effects of famine on milk volume, but quantitative data are generally lacking. Historical accounts of mothers breastfeeding during wartime sieges in Europe provide mostly anecdotal evidence of insufficient milk production among some women (Gunther, 1968). Dean (1951) reported that milk volume measured at a maternity clinic in Wuppertal, Germany, on the seventh day post partum was about 60 g lower during the war (1945-1946) than before it (1938). Severe undernutrition is widely regarded as detrimental to milk production, but there are very few supporting data.
Short-term fasting has been the subject of a few investigations. In The Gambia, Prentice et al. (1983c, 1984) reported that milk volume was unaffected in women during Ramadan, when no food or fluid is consumed from 5 a.m. to 7:30 p.m. (although intake after 7:30 p.m. may be considerable). Similarly, Neville and Oliva-Rasbach (1987) found that the rate of milk secretion was no different from the baseline among five lactating women who ate no food for 20 hours.
Strode and colleagues (1986) examined the effects of energy restriction among presumably well-nourished mothers. The experimental group reduced their energy intake by an average of 32% (range, 19 to 53%) below baseline intakes for 1 week; the control group maintained their usual intake. Among the eight mothers who restricted their intake to no less than 1,500 kcal/day, there was no reduction in milk intake by their infants, but levels of plasma prolactin tended to increase relative to those of control mothers. However, milk intakes by infants of the six mothers who decreased their energy intake below 1,500 kcal/day were reduced by an average of 15% (109 g/day) during the week after restriction had ceased (Figure 5-3). Although prolactin levels before maternal energy restriction were not correlated with milk volume, there was a correlation between change in prolactin concentration after energy restriction and subsequent milk volume. The authors concluded that the impact of longer periods of energy restriction requires further investigation.
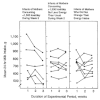
FIGURE 5-3
Change in mean milk intakes by individual infants (ages 6 to 24 weeks at week 1 of the experimental period) with change in maternal energy intake. The period of energy restriction for the mothers in the experimental group was week 2 of the 3-week experimental (more...)
In a subsequent study (Dewey et al., in press), no relationship was found between maternal weight loss1 from months 1 to 3 of lactation and milk volume at 3 months post partum; this confirmed the work of Butte et al. (1984a).
Although most of the women in these studies were not deliberately restricting their energy intake, they lost weight gradually during early lactation.
An energy deficit can also occur when energy expenditure is unusually high (in excess of energy intake). Lovelady and colleagues (1990) compared the lactation performance of eight physically fit, exercising women and eight sedentary controls. There were no significant differences in milk volume or composition despite wide group differences in energy intake and expenditure. Exercising subjects compensated for higher energy expenditure by increasing their energy intake,2 so there was no net difference in energy deficit between groups.
Energy Supplementation During Lactation and Milk Volume
Studies in rats have been conducted to investigate the effects of dietary supplementation on milk production. Rolls et al. (1980) fed rats a high-energy, low-protein supplement in addition to their usual diet during lactation. They found that although maternal food intake increased among the supplemented compared with the control animals, litter growth rate was reduced, indicating a reduction in milk output. In contrast, Roberts and Coward (1985) provided adequately fed rats with a supplement with the same protein-to-energy ratio as that of their usual diet and observed a mean increase of 31% in milk output. On average, the rats in the latter study increased both their protein and energy intakes by 20%; therefore, it is not possible to distinguish between the effects of energy and those of protein on milk volume. In a study that is more analogous to energy supplementation trials in humans, Kliewer and Rasmussen (1987) restricted rats to 50% of usual energy intake before and during pregnancy and then allowed them to feed ad libitum during lactation. Milk volume and litter growth in this group were equivalent to those of control animals.
Findings from energy supplementation studies in humans are not conclusive. Many efforts have been made over the years to ''feed the nursing mother, thereby the infant" (Sosa et al., 1976, p. 668) with mixed success. In a review of such studies up to 1980, Whitehead (1983) concludes that the results "have not been inspiring" (p. 44-45). The studies presented here are in chronologic order.
Rural Mexico. Several major investigations were conducted in Latin America and Asia during the 1960s and 1970s, but only one—a 2-year longitudinal study in a rural area of Mexico—included actual measurements of milk volume (Chávez and Martinez, 1980). In the first year of the study, one group of 17 mothers consuming their usual diet was followed throughout lactation. Milk volume was measured by the test-weighing technique in the mothers' homes for 72 consecutive hours on each of eight predetermined occasions.
In the second year of the study, another group of 17 women was followed in the same manner; however, these mothers received a food supplement providing 300 kcal and 20 g of protein per day during both pregnancy and lactation. Total mean energy intake was 2,365 kcal/day in the supplemented group, compared with the mean of 2,040 kcal/day in the unsupplemented group. (Infants in this second group were also given food supplements beginning at 3 months of age.) During the first 3 to 4 months, milk volume was 15% higher in the supplemented group than in the unsupplemented group, but the concentrations of energy, protein, and total solids in the milk were reportedly lower. Therefore, the supply of these nutrients was similar in the two groups. However, the milk sampling methods used by Chávez and Martinez (1980) were not described well enough to indicate whether the samples were representative of 24-hour production (see Chapter 6) and, thus, whether the milk energy density values were valid. Although maternal food supplementation began during pregnancy, infant weights at birth and afterward in the two groups were not compared.
The Gambia. A comprehensive food supplementation study was conducted in The Gambia between 1976 and 1982 (Prentice et al., 1983a,b). In the first phase, baseline dietary, anthropometric, and milk volume data were collected from 120 women throughout lactation. Milk volume was measured by 12-hour test weighing in the mother's home at monthly intervals and validated against 24-hour measurements for a subsample of women. In the second phase, a food supplement was provided daily to 130 women at various stages of lactation, and the same measurements were taken. The supplement provided a net increase of 723 kcal daily and approximately 57 g of protein.
Although recent findings suggest that the absolute dietary intakes were underreported in the Gambian study (Singh et al., 1989), the estimated increase in energy intake provided by the food supplementation is likely to be reasonably accurate, and it is considerably higher than that provided by most other supplementation trials. Nevertheless, Prentice et al. (1983a) found no effect of food supplementation on milk volume at any stage of lactation. Milk volume declined during the wet season despite supplementation. Although supplemented women weighed 1.8 kg (4 lb) more than unsupplemented women, averaged over the whole year, the supplemented women still lost weight during the wet season (Prentice et al., 1983b). The range of variation (standard deviation) in milk volume was the same before and after supplementation, suggesting that energy supplementation did not even increase the milk volume of women with low milk volumes. The authors concluded, in hindsight, that the nutritional status of the Gambian women prior to food supplementation was not as poor as initially believed and, therefore, that the negative results are not surprising.
In the Gambian study, levels of maternal plasma hormones (prolactin, insulin, cortisol, and triiodothyronine) decreased significantly after supplementation, suggesting a decrease in metabolic efficiency in these mothers (Prentice et al., 1983b). The women also reported fewer gastrointestinal and respiratory illnesses after food supplementation. Lack of data on energy expenditure prevent estimation of energy balance in the Gambian women. However, prolonged high prolactin levels in marginally undernourished mothers may ensure milk synthesis by channeling nutrients to the breast (Lunn et al., 1980).
Although the Gambian study indicates that milk production tends to be maintained despite a limited food supply, it did not adequately test the hypothesis that maternal food supplementation can improve milk volume because of the following limitations in study design:
- For ethical reasons, the women could not be randomized into supplemented and control groups; the entire community was included in the project both before and after the initiation of supplementation. Thus, if changes occurred over time during the study, they may have obscured any effect of the supplement.
- The sample size was relatively small at any given time post partum and at any month of the year.
- The duration of supplementation varied because supplementation was begun all at once in the entire community, and women were at different stages of reproduction.
- Milk volume was measured for only 12 hours at each sampling point; a longer period would provide a more accurate estimate.
- Infants were also supplemented from age 3 to 12 months, which may have reduced milk volume (Prentice et al., 1986).
These limitations would generally reduce the chance of finding a statistically significant effect of food supplementation on milk volume. In addition, milk sampling methods were inadequate for estimating total milk energy output. Since there is evidence of an inverse relationship between milk volume and milk energy density (Nommsen et al., in press), total milk energy output is a more useful outcome measure than milk volume alone and may produce different results.
India. A small-scale study of dietary supplementation was conducted by Girija et al. (1984) on 20 low-income lactating women in India. The subjects were provided with a supplement contributing 417 kcal of energy and 30 g of protein per day beginning 10 days or less after delivery; another 20 women served as controls. Daytime breast milk volume measured by the test-weighing procedure was no different between the groups at 1 month post partum but was higher in the supplemented group at 3 months post partum (475 g/day compared with 328 g/day). However, feeding frequency averaged seven times per day for supplemented women compared with five times per day for controls. This difference suggests that weaning may have been initiated earlier in the control group. Thus, it is difficult to interpret the results of this study.
Burma. Results of a short-term study in Burma of 21 lactating women whose weight for height was less than 80% of standard (Naing and Oo, 1987) conflict with those found in the Gambian study. The Burmese women were randomly divided into food-supplemented (N = 12) and control (N = 9) groups. The food supplement was provided twice daily for 14 days and reportedly resulted in a large net increase of approximately 900 kcal and 39 g of protein per day—even though baseline intakes (2,425 kcal/day) were not low. Average milk volume, which was measured by the test-weighing method for 12 hours on 3 consecutive days before and after supplementation, increased significantly in the supplemented group (from 662 to 787 g/day) but not in the control group. The energy content of the milk was not measured.
The differences between the findings of the Burmese and Gambian projects may reflect short-term rather than long-term effects of changes in maternal nutrition on milk volume and the selection of low weight-for-height women in the Burmese study. The randomization design of the study by Naing and Oo does permit causal inference; however, the data they present are puzzling in two respects:
- The reported 900-kcal/day increase in maternal energy intake is much larger than most other supplementation studies have been able to achieve.
- Although a large difference in milk volume was reported after supplementation, there was no difference in the mean weight gain of supplemented and control infants (perhaps because the 14-day measurement period was too short to detect this).
For these reasons, further studies should be conducted in an attempt to replicate these findings.
Energy Supplementation During Pregnancy Only
To the subcommittee's knowledge, just one study has been conducted to examine the effect of supplementation only during pregnancy on subsequent milk volume. In this study, conducted in Indonesia, 53 women provided with a high-level energy supplement (465 kcal/day) during the last trimester of pregnancy did not produce any more milk than did 55 women given a low-level energy supplement (52 kcal/day). The findings are based on test weighings of milk intake for 48 consecutive hours at 8-week intervals from 2 to 7 weeks post partum (van Steenbergen et al., 1981). The birth weights of infants in the two groups were similar (3,010 ± 291 and 3,056 ± 298 g, respectively), as were maternal weight and body mass index prior to pregnancy and at 4 weeks post partum.
The authors concluded that short-term energy supplementation was ineffective, probably because the women included in the study were not at nutritional risk. (Their reported energy intakes of 1,570 to 1,617 kcal/day were suggestive of undernutrition, but their body mass index at 4 weeks post partum averaged 19, which is considered to be at the low end of the normal range.) Mean milk intakes of the exclusively breastfed infants in this population (686 to 830 g/day) were very similar to those of infants in industrialized countries.
Maternal Energy Reserves and Milk Volume
In theory, the energy stored as fat deposits during pregnancy is used to support milk production post partum, but there are few data with which to evaluate this relationship. Studies in animals have provided no consistent evidence of poor lactation performance resulting from poor prenatal nutrition alone (Kliewer and Rasmussen, 1987). Compared with controls, there was no statistically significant decrease in milk yield or litter weight at day 14 among rats fed 50% of ad libitum intakes before and during pregnancy but allowed to eat ad libitum during lactation. In cross-sectional studies of relatively well-nourished women in the United States, correlations between infant milk intake during the first 5 months post partum and maternal prepregnancy weight or pregnancy weight gain were not statistically significant (Butte et al., 1984a; Dewey et al., 1986). Although the range in prepregnancy weight for height was relatively wide (76 to 153% of desirable body weight) in these studies, very few of the women gained less than 11 kg (24 lb) during pregnancy.
In Indonesia, prepregnancy maternal body mass index was positively associated with milk intake of breastfed infants at 18 to 22 weeks post partum but not at 2 to 6, 10 to 14, or 26 to 30 weeks (controlling for infant birth weight did not change this association) (van Steenbergen et al., 1989). Few data have been reported in developing countries on the potential influence of pregnancy weight gain. Because maternal nutritional status (as indicated by weight for height) during pregnancy is strongly associated with nutritional status post partum, it is not possible to examine separately the influence of prenatal maternal body composition.
Mixed results have been obtained from observational studies of associations between indices of relative weight (such as body mass index) during lactation and milk volume in human populations. In industrialized countries, milk volume and maternal anthropometric variables have not been associated (Butte et al., 1984a; Dewey et al., 1986; Prentice et al., 1986). In developing countries, the situation is more complex. In an apparently undernourished population in Bangladesh, Brown et al. (1986b) found no statistically significant association between infant milk intake and maternal anthropometric variables, but they did report that milk energy output (the total energy value of the milk produced) was higher in women with greater arm circumference and triceps skinfold measurements. In that analysis, no adjustments were made for the potential influence of infant weight. However, in an analysis of data collected at different times for the same individuals, there was a significant association between increase in maternal body weight and increased milk volume even when the analysis was controlled for infant weight. Curiously, these mothers tended to gain weight during the first 3 months post partum, despite producing an average milk volume of 550 to 690 g/day.
In The Gambia, milk volume during the baseline period (before food supplementation) was positively correlated with maternal weight and height, but not when infant weight was included in the analysis (Prentice et al., 1986). In an earlier study of 16 lactating women in The Gambia, milk volume measured by 12-hour test weighing was inversely related to increases in maternal triceps and subscapular skinfold thicknesses during weeks 6 to 12 of lactation (Paul et al., 1979). This surprising finding was interpreted by the authors to indicate that there is competition between milk synthesis and replenishment of maternal fat stores during lactation.
The findings of other observational studies of maternal weight for height and milk volume are often difficult to interpret because they include infants who are not exclusively breastfed. For example, van Steenbergen et al. (1983) compared lactation performance of low weight-for-height women (70 to 80% of expected) in Kenya with the performance of normal-weight women (90 to 115% of expected) during the first 6 months post partum. In the low weight-for-height group, milk volume was found to be lower (695 compared with 790 g/day), but milk energy density was approximately 12% higher. In both groups, most infants were given supplementary foods, usually beginning in the third month. No data comparing only the exclusively breastfed infants were provided. In studies of mixed-fed infants, one cannot determine retrospectively whether low milk volume leads mothers to supplement early or whether early supplementation leads to low milk volumes.
The complaint of having an insufficient supply of milk for the baby is heard in both well-nourished and poorly nourished populations, but the incidence of this complaint was not related to maternal nutritional status in cross-cultural studies conducted by Tully and Dewey (1985).
Energy Utilization During Lactation
There are several potential mechanisms of energy conservation during lactation in addition to the mobilization and utilization of fat. Prentice and Prentice (1988) described three such "energy-sparing adaptations" that may permit lactation to proceed normally when energy intake is limited, including decreases in basal metabolic rate (BMR), thermogenesis, and physical activity. Whether these types of decreases should be considered adaptations is a matter of considerable debate.
There is conflicting evidence regarding whether BMR increases, decreases, or stays constant during lactation. Since the energy cost of milk synthesis is included within the BMR, one might predict BMR to increase slightly during lactation. Sadurskis et al. (1988) calculated an approximately 5.6% increase in BMR over prepregnancy values in the same women at 2 months of lactation, correcting for changes in body composition. Earlier studies in India also showed higher BMRs among lactating women than among nonlactating, "normal" Indian women (Khan and Belavady, 1973; Venkatachalam and Gopalan, 1960). In contrast, other investigators found lower BMRs during lactation than expected on the basis of weight and height (Blackburn and Calloway, 1985; Lovelady et al., 1990; Prentice and Whitehead, 1987; van Raaij et al., 1987) or no difference between lactating and nonlactating women (Illingworth et al., 1986; Schutz et al., 1980).
A 30% reduction in postprandial thermogenesis during lactation was observed in one study (Illingworth et al., 1986). This has little overall impact, however, since postprandial thermogenesis represents only about 10% of total energy expenditure, and thus, only about 60 kcal is saved per day.
There is considerable variation in the degree to which energy expenditure for activity could be reduced during lactation. During the postpartum period, the demands of feeding and caring for an infant take up considerable time but may require either more or less energy than the woman's former activities. Total activity levels of lactating mothers may be constrained by being housebound with a new baby. Data from small samples of relatively sedentary lactating women in the United States indicate that total energy expenditure (not including milk production) averages only 1,800 to 1,900 kcal/day (Blackburn and Calloway, 1976; Lovelady et al., 1990); the estimated expenditure for nonlactating women, assuming light to moderate activity, is 2,200 kcal/day (NRC, 1989). In contrast, lactating women exercising on a regular basis expended an average of 2,631 kcal/day, not including energy output in milk (Lovelady et al., 1990). Women who must care for several children or who are employed in physically demanding jobs may also have high activity levels. Mothers who do not have access to adequate food cannot always decrease their workload to reduce their energy deficit (Singh et al., 1989). If these mothers decrease other day-to-day activities as an energy-sparing mechanism, there may be adverse effects on their quality of life.
Maternal Protein Intake
Studies in lactating rats (Jansen and Monte, 1977; Naismith et al., 1982; Sampson and Jansen, 1985) and in swine (Mahan, 1977) indicate that protein intake can increase milk volume independently of total energy intake. Early studies in humans by Gopalan (1958) and Edozien et al. (1976) suggest the same relationship: milk output of women in India and Nigeria increased when protein intake was increased from 50 to 60 g/day to approximately 100 g/day. Increasing protein above 100 g/day caused no further change in milk volume. In both studies, however, the samples were very small (N = 6 and 8, respectively), there were no control groups, and their designs may have biased the results. In the Indian study, protein intake was increased in stages from 61 to 99 to 114 g/day in three successive 10-day periods, and milk samples were obtained by complete expression of both breasts at the 10 a.m. feeding during the last 3 days of each period. Because milk intake may increase with the age of the infants and repeated expression of extra milk may stimulate greater milk production (Dewey and Lönnerdal, 1986), milk volumes may have increased during the study for these reasons rather than in response to increased maternal protein intake. In the Nigerian study, total milk volume was calculated by measuring infant intake and by pumping residual milk after each feeding for each of the 7-day measurement periods. The extra stimulation provided by removing residual milk may have increased milk production.
In two small-scale studies of well-nourished women whose usual protein intake ranged from approximately 80 to 100 g/day, no statistically significant changes in milk volume were observed when protein intake was reduced to 8% of total calories or increased to 20% of total calories for 4 days (Forsum and Lönnerdal, 1980) or when daily protein intake was either 1.0 or 1.5 g/kg of body weight for 7 to 10 days (Motil et al., 1986). However, the very small sample sizes (N = 3 and 15, respectively) and short duration of these studies preclude any definitive conclusions regarding the impact of varying protein intake.
Fluid Intake
It is widely assumed that milk production requires a high fluid intake on the part of the mother, yet the evidence suggests that lactating women can tolerate a considerable amount of water restriction and that supplemental fluids have little effect on milk volume. Lactating women who consumed no food or fluids from 5:00 a.m. to 7:30 p.m. during Ramadan lost 7.6% of their total body water and experienced increases in serum indices of dehydration, although values remained within the normal range (Prentice et al., 1984). Milk volume was unaffected, but changes in milk composition (lower lactose concentrations; increased osmolality due to higher electrolyte concentrations) indicated alterations in mammary cell permeability. Water turnover was very high, in part because the women apparently superhydrated themselves overnight prior to the fasting period.
Two early studies from Germany (Olsen, 1941) and France (Lelong et al., 1949) also showed no influence on milk output when fluid intake ranged from 600 to 2,775 ml/day during 3- to 4-day periods or when total fluid intake from all sources was restricted to 1,765 ml/day for 10 days. Dusdieker et al. (1985) examined the effect produced by increasing fluid intake by at least 25% for 3 days among 21 lactating women in the United States and found no change in milk volume and no correlation between fluid intake and milk volume (Figure 5-4).
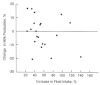
FIGURE 5-4
Relationship between increase in maternal fluid intake for 3 days and percent change in milk production. From Dusdieker et al. (1985) with permission.
In an earlier study, Illingworth and Kilpatrick (1953) asked 104 lactating women to drink at least 2,880 ml of liquid per day (high-fluids group) and 106 control women to drink as much as desired. In the first 9 days post partum, actual fluid intake averaged approximately 3,200 ml/day in the high-fluids group and about 2,100 ml/day in the control group. Neither infant growth in the first month nor duration of breastfeeding differed between groups. Milk intake at a test feed on the eighth day post partum tended to be lower in the high-fluids group. The authors thus cautioned against drinking fluids in excess of natural thirst inclination. However, thirst may sometimes function too slowly to prevention dehydration among women with high fluid losses resulting from exercise or high ambient temperature (experienced by many women without air conditioning in the summer). Thus, careful attention to adequacy of fluid intake is warranted in such situations, but under most conditions there appears to be no justification for emphasizing high fluid intake as a way to improve milk production.
Conclusions
- Studies conducted in the United States, other developed countries, and developing countries indicate that the average level of milk production is approximately 750 to 800 ml/day in women with widely varying dietary intakes and with varying nutritional status, as measured by weight and skinfold thickness.
- Potential production of milk by lactating women appears to be considerably higher than actual intakes by single infants, as indicated by the high milk volumes produced by women nursing twins or even triplets.
- Factors other than maternal nutrition affect milk volume and should be considered in any evaluation of lactation performance. Maternal age and parity appear to have little, if any, influence, but variables such as maternal stress and the nursing behavior of both mother and infant are potentially important.
- Maternal nutritional status, as measured by anthropometric indices prenatally or post partum, is not related to milk volume in studies conducted in industrialized countries such as the United States. In other words, infants of thin women generally consume as much breast milk as infants of normal-weight or overweight women. In less developed countries, the results are mixed; some studies show a positive association between maternal body composition (adiposity) and milk volume.
- Average milk volumes of lactating women are comparable in industrialized and developing countries, despite substantial differences in energy and nutrient intake. This suggests that maternal energy intake is not strongly associated with milk volume. Studies in animals indicate that there may be a threshold below which energy intake is insufficient to support normal milk production; it is likely that most studies in humans have been conducted in groups with intakes above this hypothesized threshold.
- Food supplementation of lactating women in areas where malnutrition is prevalent has generally had little, if any, impact on milk volume. However, such supplementation may improve maternal health and therefore is more likely to benefit the mother than the infant, except in cases in which milk composition is affected (see Chapter 6).
- It is customary to lose weight gradually during lactation. In the United States, lactating women tend to be heavier than their ideal body weight immediately post partum, and some successfully lose up to 2 kg (˜4.5 lb) per month with no apparent deleterious effects on milk production.
- Women who exercise regularly appear to produce an adequate volume of milk.
- The influence of maternal intake of specific nutrients on milk volume has not been investigated thoroughly. Early studies in developing countries show an association of protein intake with milk volume, but limitations of the study designs prohibit definitive conclusions.
- Adequate fluid intake during lactation is desirable to maintain maternal health, but supplemental fluids consumed in excess of natural thirst have no effect on milk volume.
Recommendations For Clinical Practice
- Advise women that the average rate of weight loss post partum (0.5 to 1.0 kg, or 1 to 2 lb, per month after the first month) appears to be consistent with maintaining adequate milk volume. If a lactating woman is overweight, a weight loss of up to 2 kg (˜4.5 lb) per month is unlikely to adversely affect milk volume, but such women should be alert for any indications that the infant's appetite is not being satisfied. Rapid weight loss (>2 kg/month after the first month post partum) is not advisable for breastfeeding women.
- The level of physical activity needs to be considered when advising women about adequacy of energy intake during lactation. Intakes below 1,500 kcal/day are not recommended at any time during lactation, although brief fasts (lasting less than 1 day) are unlikely to decrease milk volume. Liquid diets and weight loss medications are not recommended.
- Since the impact of curtailing maternal energy intake during the first 2 to 3 weeks post partum is unknown, dieting during this period is not recommended.
- If alcohol is used, advise the lactating woman to limit her intake to no more than 0.5 g of alcohol per kg of maternal body weight per day. Intake over this level may impair the milk ejection reflex. For a 60-kg (132-lb) woman, 0.5 g of alcohol per kg of body weight corresponds to approximately 2 to 2.5 oz of liquor, 8 oz of table wine, or 2 cans of beer.
- Actively discourage cigarette smoking among lactating women, not only because it may reduce milk volume but because of its other harmful effects on the mother and her infant.
- Discourage intake of large quantities of coffee, other caffeine-containing beverages and medications, and decaffeinated coffee.
- Because the early management of lactation has a strong influence on the establishment of an adequate milk supply, breastfeeding guidance should be provided prenatally and continued in the hospital after delivery and during the early postpartum period.
- Promote breastfeeding practices that are responsive to the infant's natural appetite. In the first few weeks, infants should nurse at least 8 times per day, and some may nurse as often as 15 or more times per day. After the first month, infants fed on demand usually nurse 5 to 12 times per day.
References
- Andersen, A.N., C. Lund-Andersen, J.F. Larsen, N.J. Christensen, J.J. Legros, F. Louis, H. Angelo, and J. Molin. 1982. Suppressed prolactin but normal neurophysin levels in cigarette smoking breastfeeding women. Clin. Endocrinol. 17:363-368. [PubMed: 7139967]
- Blackburn, M.W., and D.H. Calloway. 1976. Energy expenditure and consumption of mature, pregnant and lactating women. J. Am. Diet. Assoc. 69:29-37. [PubMed: 932372]
- Blackburn, M.W., and D.H. Calloway. 1985. Heart rate and energy expenditure of pregnant and lactating women. Am. J. Clin. Nutr. 42:1161-1169. [PubMed: 4072951]
- Blake, C.A., and C.H. Sawyer. 1972. Nicotine blocks the suckling-induced rise in circulating prolactin in lactating rats. Science 177:619-621. [PubMed: 5065617]
- Brown, K.H., R.E. Black, A.D. Robertson, N.A. Akhtar, G. Ahmed, and S. Becker. 1982. Clinical and field studies of human lactation: methodological considerations. Am. J. Clin. Nutr. 35:745-756. [PubMed: 7072626]
- Brown, K.H., A.D. Robertson, and N.A. Akhtar. 1986. a. Lactational capacity of marginally nourished mothers: infants' milk nutrient consumption and patterns of growth. Pediatrics 78:920-927. [PubMed: 3763305]
- Brown, K.H., N.A. Akhtar, A.D. Robertson, and M.G. Ahmed. 1986. b. Lactational capacity of marginally nourished mothers: relationships between maternal nutritional status and quantity and proximate composition of milk. Pediatrics 78:909-919. [PubMed: 3763304]
- Brown, K.H., R.Y. Stallings, H. Creed de Kanashiro, G. Lopez de Romaña; and R.E. Black. In press. Effects of common illnesses on infants' energy intakes from breast milk and other foods during longitudinal community-based studies in Huascar (Lima), Peru. Am. J. Clin. Nutr. [PubMed: 2239775]
- Butte, N.F., and D.H. Calloway. 1981. Evaluation of lactational performance of Navajo women. Am. J. Clin. Nutr. 34:2210-2215. [PubMed: 7293949]
- Butte, N.F., and C. Garza. 1985. Energy and protein intakes of exclusively breastfed infants during the first four months of life. Pp. 63-83 in M. Gracey, editor; and F. Falkner, editor. , eds. Nutritional Needs and Assessment of Normal Growth. Raven Press, New York.
- Butte, N.F., C. Garza, J.E. Stuff, E.O. Smith, and B.L. Nichols. 1984. a. Effect of maternal diet and body composition on lactational performance. Am. J. Clin. Nutr. 39:296-306. [PubMed: 6695830]
- Butte, N.F., C. Garza, E.O. Smith, and B.L. Nichols. 1984. b. Human milk intake and growth in exclusively breastfed infants. J. Pediatr. 104:187-195. [PubMed: 6694010]
- Butte, N.F., W.W. Wong, B.W. Patterson, C. Garza, and P.D. Klein. 1988. Human-milk intake measured by administration of deuterium oxide to the mother: a comparison with the test-weighing technique. Am. J. Clin. Nutr. 47:815-821. [PubMed: 2834941]
- Casey, C.E., M.R. Neifert, J.M. Seacat, and M.C. Neville. 1986. Nutrient intake by breastfed infants during the first five days after birth. Am. J. Dis. Child. 140:933-936. [PubMed: 3740001]
- Chandra, R.K. 1981. Breastfeeding, growth and morbidity. Nutr. Res. 1:25-31.
- Chávez, A., and C. Martinez. 1980. Effects of maternal undernutrition and dietary supplementation on milk production. Pp. 274-284 in H. Aebi, editor; and R. Whitehead, editor. , eds. Maternal Nutrition During Pregnancy and Lactation. Hans Huber, Bern.
- Cobo, E. 1973. Effect of different doses of ethanol on the milk-ejecting reflex in lactating women. Am. J. Obstet. Gynecol. 115:817-821. [PubMed: 4688584]
- Coward, W.A., T.J. Cole, H. Guber, S.B. Roberts, and I. Fleet. 1982. Water turnover and measurement of milk intake. Pflugers. Arch. 393:344-347. [PubMed: 7122210]
- Coward, W.A., A.A. Paul, and A.M. Prentice. 1984. The impact of malnutrition on human lactation: observations from community studies. Fed. Proc., Fed. Am. Soc. Exp. Biol. 43:2432-2437. [PubMed: 6427014]
- Cross, B.A. 1955. The hypothalamus and the mechanism of sympathetico-adrenal inhibition of milk ejection. J. Endocrinol. 12:15-28. [PubMed: 14354100]
- Dean, R.F.A. 1951. The size of the baby at birth and the yield of breast milk. Med. Res. Counc. (G. B.), Spec. Rep. Ser. 275:346-378. [PubMed: 14866615]
- de Carvalho, M., S. Robertson, R. Merkatz, and M. Klaus. 1982. Milk intake and frequency of feeding in breastfed infants. Early Hum. Dev. 7:155-163. [PubMed: 7151727]
- de Carvalho, M., S. Robertson, A. Friedman, and M. Klaus. 1983. Effect of frequent breastfeeding on early milk production and infant weight gain. Pediatrics 72:307-311. [PubMed: 6889034]
- de Carvalho, M., D.M. Anderson, A. Giangreco, and W.B. Pittard III. 1985. Frequency of milk expression and milk production by mothers of nonnursing premature neonates. Am. J. Dis. Child. 139:483-485. [PubMed: 3984973]
- Dewey, K.G., and M.J. Heinig. 1987. Does 4-day test-weighing to determine breast milk intake affect nursing frequency? Fed. Proc., Fed. Am. Soc. Exp. Biol. 46:571.
- Dewey, K.G., and B. Lönnerdal. 1983. Milk and nutrient intake of breastfed infants from 1 to 6 months: Relation to growth and fatness. J. Pediatr. Gastroenterol. Nutr. 2:497-506. [PubMed: 6620057]
- Dewey, K.G., and B. Lönnerdal. 1986. Infant self-regulation of breast milk intake. Acta Paediatr. Scand. 75:893-898. [PubMed: 3564971]
- Dewey, K.G., D.A. Finley, and B. Lönnerdal. 1984. Breast milk volume and composition during late lactation (7-20 months). J. Ped. Gastroenterol. Nutr. 3:713-720. [PubMed: 6502372]
- Dewey, K.G., D.A. Finley, M.A. Strode, and B. Lönnerdal. 1986. Relationship of maternal age to breast milk volume and composition. Pp. 263-273 in M. Hamosh, editor; and A.S. Goldman, editor. , eds. Human Lactation 2: Maternal and Environmental Factors. Plenum Press, New York.
- Dewey, K.G., M.J. Heinig, L.A. Nommsen, and B. Lönnerdal. In press. Maternal vs. infant factors related to breast milk intake and residual milk volume: the DARLING study. Pediatrics. [PubMed: 2034486]
- Dusdieker, L.B., B.M. Booth, P.J. Stumbo, and J.M. Eichenberger. 1985. Effect of supplemental fluids on human milk production. J. Pediatr. 106:207-211. [PubMed: 3968608]
- Edozien, J.C., M.A.R. Khan, and C.I. Waslien. 1976. Human protein deficiency: results of a Nigerian village study. J. Nutr. 106:312-328.
- Egli, G.E., N.S. Egli, and M. Newton. 1961. The influence of the number of breastfeedings on milk production. Pediatrics 27:314-317. [PubMed: 13725927]
- Feher, S.D.K., L.R. Berger, J.D. Johnson, and J.B. Wilde. 1989. Increasing breast milk production for premature infants with a relaxation/imagery audiotape. Pediatrics 83:57-60. [PubMed: 2642620]
- Ferry, J.D., B.K. McLean, and M.B. Nikitovitch-Winer. 1974. Tobacco-smoke inhalation delays suckling-induced prolactin release in the rat. Proc. Soc. Exp. Biol. Med. 147:110-113. [PubMed: 4474667]
- Fjeld, C.R., K.H. Brown, and D.A. Schoeller. 1988. Validation of the deuterium oxide method for measuring average daily milk intake in infants. Am. J. Clin. Nutr. 48:671-679. [PubMed: 2843027]
- Ford, K., and M. Labbok. 1987. Contraceptive usage during lactation in the United States: an update. Am. J. Public Health 77:79-81. [PMC free article: PMC1646818] [PubMed: 3789244]
- Forsum, E., and B. Lönnerdal. 1980. Effect of protein intake on protein and nitrogen composition of breast milk. Am. J. Clin. Nutr. 33:1809-1813. [PubMed: 7405883]
- Fuchs, A., and G. Wagner. 1963. The effect of ethyl alcohol on the release of oxytocin in rabbits. Acta Endocrinol. 44:593-605. [PubMed: 14082547]
- Girija, A., P. Geervani, and R.G. Rao. 1984. Influence of dietary supplementation during lactation on lactation performance. J. Trop. Pediatr. 30:140-144. [PubMed: 6737552]
- Goldfarb, J., and E. Tibbetts. 1989. Breastfeeding Handbook: A Practical Reference for Physicians, Nurses, and Other Health Professionals. Enslow, Hillside, N.J. 256 pp.
- Gopalan, C. 1958. Studies on lactation in poor Indian communities. J. Trop. Pediatr. 4:87-97. [PubMed: 13611813]
- Hamosh, M., M.R. Simon, and P. Hamosh. 1979. Effect of nicotine on the development of fetal and suckling rats. Biol. Neonate 35:290-297. [PubMed: 476202]
- Hofvander, Y., U. Hagman, C. Hillervik, and S. Sjölin. 1982. The amount of milk consumed by 1-3 months old breast- or bottle-fed infants. Acta Paediatr. Scand. 71:953-958. [PubMed: 7158334]
- Hopkinson, J.M., R.J. Schanler, and C. Garza. 1988. Milk production by mothers of premature infants. Pediatrics 81:815-820. [PubMed: 3368280]
- Horrobin, D. 1979. Prolactin. Annual Research Reviews, X. Vol. 7. Eden Press, St. Albans, Vt. 126 pp.
- Hytten, F.E. 1954. Clinical and chemical studies in human lactation. VIII. Relationship of the age, physique, and nutritional status of the mother to the yield and composition of her milk. Br. Med. J. 2:844-845. [PMC free article: PMC2079482] [PubMed: 13199324]
- Hytten, F.E., and A.M. Thomson. 1961. Nutrition of the lactating woman. Pp. 3-46 in Kon, S.K., editor; , and A.T. Cowie, editor. , eds. Milk: the Mammary Gland and Its Secretion. Academic Press, New York.
- Illingworth, R.S., and B. Kilpatrick. 1953. Lactation and fluid intake. Lancet 2:1175-1177.
- Illingworth, P.J., R.T. Jung, P.W. Howie, P. Leslie, and T.E. Isles. 1986. Diminution in energy expenditure during lactation. Br. Med. J. 292:437-441. [PMC free article: PMC1339421] [PubMed: 3081114]
- IOM (Institute of Medicine). 1990. Nutrition During Pregnancy: Weight Gain and Nutrient Supplements. Report of the Subcommittee on Nutritional Status and Weight Gain During Pregnancy, Subcommittee on Dietary Intake and Nutrient Supplements During Pregnancy, Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board. National Academy Press, Washington, D.C. 468 pp.
- Janas, L.M., and M.F. Picciano. 1986. Quantities of amino acids ingested by human milk-fed infants. J. Pediatr. 109:802-807. [PubMed: 3772659]
- Janas, L.M., M.F. Picciano, and T.F. Hatch. 1985. Indices of protein metabolism in term infants fed human milk, whey-predominant formula, or cow's milk formula. Pediatrics 75:775-784. [PubMed: 3872443]
- Jansen, G.R., and W.C. Monte. 1977. Amino acid fortification of bread fed at varying levels during gestation and lactation in rats. J. Nutr. 107:300-309. [PubMed: 401878]
- Jansen, A.A.J., R. Luyken, S.H. Malcom, and J.J.L. Willems. 1960. Quantity and composition of breast milk in Baik Island (Neth. New Quinea). Trop. Geog. Med. 2:138-144.
- Kaucher, M., E.Z. Moyer, A.J. Richards, H.H. Williams, A.L. Wertz, and I.G. Macy. 1945. Human milk studies. XX. The diet of lactating women and the collection and preparation of food and human milk for analysis. Am. J. Dis. Child. 70:142-147. [PubMed: 21004400]
- Khan, L., and B. Belavady. 1973. Basal metabolism in pregnant and nursing women and children. Indian J. Med. Res. 61:1853-1860. [PubMed: 4787042]
- Kliewer, R.L., and K.M. Rasmussen. 1987. Malnutrition during the reproductive cycle: effects on galactopoietic hormones and lactational performance in the rat. Am. J. Clin. Nutr. 46:926-935. [PubMed: 3687825]
- Koetsawang, S. 1987. The effects of contraceptive methods on the quality and quantity of breast milk. Int. J. Gynaecol. Obstet. 25 Suppl.:115-127. [PubMed: 2892711]
- Lawrence, R.A. 1985. Breastfeeding: A Guide for the Medical Profession, 2nd ed. C.V. Mosby, St. Louis. 601 pp.
- Lawrence, R.A. 1989. Breastfeeding: A Guide for the Medical Profession, 3rd ed. C.V. Mosby, St. Louis. 652 pp.
- Lelong, M., F. Alison, and J. Vinceneux. 1949. Secretion lactee humaine et alimentation hydrique. Lait 29:237-246.
- Lipsman, S., K.G. Dewey, and B. Lönnerdal. 1985. Breastfeeding among teenage mothers: milk composition, infant growth, and maternal dietary intake. J. Pediatr. Gastroenterol. Nutr. 4:426-434. [PubMed: 4020574]
- Lönnerdal, B. 1986. Effect of oral contraceptives on lactation. Pp. 453-465 in M. Hamosh, editor; and A.S. Goldman, editor. , eds. Human Lactation 2: Maternal and Environmental Factors. Plenum Press, New York.
- Lönnerdal, B., E. Forsum, M. Gebre-Medhin, and L. Hambraeus. 1976. Breast milk composition in Ethiopian and Swedish mothers. II. Lactose, nitrogen. and protein contents. Am. J. Clin. Nutr. 29:1134-1141. [PubMed: 973602]
- Lovelady, C.A., B. Lönnerdal, and K.G. Dewey. 1990. Lactation performance of exercising women. Am. J. Clin. Nutr. 52:103-109. [PubMed: 2360539]
- Lunn, P.G., A.M. Prentice, S. Austin, and R.G. Whitehead. 1980. Influence of maternal diet on plasma-prolactin levels during lactation. Lancet 1:623-625. [PubMed: 6102628]
- Lunn, P.G., S. Austin, A.M. Prentice, and R.G. Whitehead. 1984. The effect of improved nutrition on plasma prolactin concentrations and postpartum infertility in lactating Gambian women. Am. J. Clin. Nutr. 39:227-235. [PubMed: 6695825]
- Lyon, A.J. 1983. Effects of smoking on breastfeeding. Arch. Dis. Child. 58:378-380. [PMC free article: PMC1627880] [PubMed: 6859920]
- Macy, I.G., H.A. Hunscher, E. Donelson, and B. Nims. 1930. Human milk flow. Am. J. Dis. Child. 39:1186-1204.
- Mahan, D.C. 1977. Effect of feeding various gestation and lactation dietary protein sequences on long-term reproductive performance in swine. J. Anim. Sci. 45:1061-1072. [PubMed: 599096]
- Martin, R.H. 1983. The place of PRL in human lactation. Clin. Endocrinol. 18:295-299. [PubMed: 6861368]
- Matheson, I., and G.N. Rivrud. 1989. The effect of smoking on lactation and infantile colic. J. Am. Med. Assoc. 26:42-43. [PubMed: 2908985]
- Moore, B.J., T. Gerardo-Gettens, B.A. Horwitz, and J.S. Stern. 1986. Hyperprolactinemia stimulates food intake in the female rat. Brain Res. Bull. 17:563-569. [PubMed: 3779456]
- Motil, K.J., C.M. Montandon, and C. Garza. 1986. Effect of dietary protein intake on milk production in lactating women. Am. J. Clin. Nutr. 43:677.
- NRC (National Research Council). 1989. Recommended Dietary Allowances, 10th ed. Report of the Subcommittee on the Tenth Edition of the RDAs, Food and Nutrition Board, Commission on Life Sciences. National Academy Press, Washington, D.C. 284 pp.
- Naing, K.M., and T.T. Oo. 1987. Effect of dietary supplementation on lactation performance of undernourished Burmese mothers. Food Nutr. Bull. 9:59-61.
- Naismith, D.J., D.P. Richardson, and A.E. Pritchard. 1982. The utilization of protein and energy during lactation in the rat, with particular regard to the use of fat accumulated in pregnancy. Br. J. Nutr. 48:433-441. [PubMed: 7115665]
- Neifert, M.R., and J.M. Seacat. 1986. Mammary gland anomalies and lactation failure. Pp. 293-299 in M. Hamosh, editor; and A.S. Goldman, editor. , eds. Human Lactation 2: Maternal and Environmental Factors. Plenum Press, New York.
- Neifert, M.R., S.L. McDonough, and M.C. Neville. 1981. Failure of lactogenesis associated with placental retention. Am. J. Obstet. Gynecol. 140:477-478. [PubMed: 7246673]
- Neville, M.C., editor; , and M.R. Neifert, editor. , eds. 1983. Lactation: Physiology, Nutrition, and Breast-Feeding. Plenum Press, New York. 466 pp.
- Neville, M., and J. Oliva-Rasbach. 1987. Is maternal milk production limiting for infant growth during the first year of life in breastfed infants? Pp. 123-133 in A.S. Goldman, editor; , S.A. Atkinson, editor; , and L.A. Hanson, editor. , eds. Human Lactation 3: The Effects of Human Milk on the Recipient Infant. Plenum Press, New York.
- Neville, M.C., R. Keller, J. Seacat, V. Lutes, M. Neifert, C. Casey, J. Allen, and P. Archer. 1988. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 48:1375-1386. [PubMed: 3202087]
- Newton, M., and N.R. Newton. 1948. The let-down reflex in human lactation. J. Pediatr. 33:698-704. [PubMed: 18101061]
- Newton, N.R., and M. Newton. 1950. Relation of the let-down reflex to the ability to breastfeed. Pediatrics 5:726-733. [PubMed: 15417272]
- Noel, G.L., H.K. Suh, and A.G. Frantz. 1974. Prolactin release during nursing and breast stimulation in postpartum and nonpostpartum subjects. J. Clin. Endocrinol. Metab. 38:413-423. [PubMed: 4815172]
- Nommsen, L.A., M.J. Heinig, B. Lönnerdal, and K.G. Dewey. 1989. Appropriate timing of complementary feeding of breastfed infants. FASEB J. 3:A10-54 (abstract).
- Nommsen, L.A., C.A. Lovelady, M.J. Heinig, B. Lönnerdal, and K.G. Dewey. In press. Determinants of energy, protein, lipid and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. [PubMed: 1989413]
- Olsen, A. 1941. Om diegivningsevnen under torst og under extradrikning. Ugeskr. Laeg. 103:897-905.
- Pao, E.M., J.M. Himes, and A.F. Roche. 1980. Milk intakes and feeding patterns of breastfed infants. J. Am. Diet. Assoc. 77:540-545. [PubMed: 7430508]
- Paul, A.A., E.M. Muller, and R.G. Whitehead. 1979. The quantitative effects of maternal dietary energy intake on pregnancy and lactation in rural Gambian women. Trans. R. Soc. Trop. Med. Hyg. 73:686-692. [PubMed: 538811]
- Picciano, M.F., E.J. Calkins, J.R. Garrick, and R.H. Deering. 1981. Milk and mineral intakes of breastfed infants. Acta Paediatr. Scand. 70:189-194. [PubMed: 7234402]
- Pollitt, E., M. Gilmore, and M. Valcarcel. 1978. The stability of sucking behavior and its relationship to intake during the first month of life. Infant Behav. Dev. 1:347-357.
- Prentice, A.M. 1980. Variations in maternal dietary intake, birth weight and breast-milk output in The Gambia. Pp. 167-183 in Aebi, H., editor; and R.G. Whitehead, editor. , eds. Maternal Nutrition During Pregnancy and Lactation. Hans Huber Publishers, Bern, Switzerland.
- Prentice, A. 1986. The effect of maternal parity on lactational performance in a rural African community. Pp. 165-173 in M. Hamosh, editor; and A.S. Goldman, editor. , eds. Human Lactation 2: Maternal and Environmental Factors. Plenum Press, New York.
- Prentice, A.M., and A. Prentice. 1988. Energy costs of lactation. Annu. Rev. Nutr. 8:63-79. [PubMed: 3060180]
- Prentice, A.M., and R.G. Whitehead. 1987. The energetics of human reproduction. Symp. Zool. Soc. London: 57:275-304.
- Prentice, A.M., S.B. Roberts, A. Prentice, A.A. Paul, M. Watkinson, A.A. Watkinson, and R.G. Whitehead. 1983. a. Dietary supplementation of lactating Gambian women. I. Effect on breast-milk volume and quality. Hum. Nutr.: Clin. Nutr. 37C:53-64. [PubMed: 6341320]
- Prentice, A.M., P.G. Lunn, M. Watkinson, and R.G. Whitehead. 1983. b. Dietary supplementation of lactating Gambian women. II. Effect on maternal health, nutritional status and biochemistry. Hum. Nutr.: Clin. Nutr. 37C:65-74. [PubMed: 6841136]
- Prentice, A.M., A. Prentice, W.H. Lamb, P.G. Lunn, and S. Austin. 1983. c. Metabolic consequences of fasting during Ramadan in pregnant and lactating women. Hum. Nutr.: Clin. Nutr. 37C:283-294. [PubMed: 6643131]
- Prentice, A.M., W.H. Lamb, A. Prentice, and W.A. Coward. 1984. The effect of water abstention on milk synthesis in lactating women. Clin. Sci. 66:291-298. [PubMed: 6692661]
- Prentice, A., A. Paul, A. Prentice, A. Black, T. Cole, and R. Whitehead. 1986. Cross-cultural differences in lactational performance. Pp. 13-44 in M. Hamosh, editor; and A.S. Goldman, editor. , eds. Human Lactation 2: Maternal and Environmental Factors. Plenum Press, New York.
- Rattigan, S., A.V. Ghisalberti, and P.E. Hartmann. 1981. Breast-milk production in Australian women. Br. J. Nutr. 45:243-249. [PubMed: 7213579]
- Roberts, S.B., and W.A. Coward. 1985. Dietary supplementation increases milk output in the rat. Br. J. Nutr. 53:1-9. [PubMed: 4063254]
- Roberts, S.B., T.J. Cole, and W.A. Coward. 1985. Lactational performance in relation to energy intake in the baboon. Am. J. Clin. Nutr. 41:1270-1276. [PubMed: 4003332]
- Rolls, B.J., E.A. Rowe, S.E. Fahrbach, L. Agius, and D.H. Williamson. 1980. Obesity and high energy diets reduce survival and growth rates of rat pups. Proc. Nutr. Soc. 39:51A. [PubMed: 7403088]
- Sadurskis, A., N. Kabir, J. Wager, and E. Forsum. 1988. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. Am. J. Clin. Nutr. 48:44-49. [PubMed: 3389329]
- Saint, L., M. Smith, and P.E. Hartmann. 1984. The yield and nutrient content of colostrum and milk of women giving birth to 1 month post-partum. Br. J. Nutr. 52:87-95. [PubMed: 6743645]
- Saint, L., P. Maggiore, and P.E. Hartmann. 1986. Yield and nutrient content of milk in eight women breastfeeding twins and one woman breastfeeding triplets. Br. J. Nutr. 56:49-58. [PubMed: 3676208]
- Salariya, E.M., P.M. Easton, and J.I. Cater. 1978. Duration of breastfeeding after early initiation and frequent feeding. Lancet 2:1141-1143. [PubMed: 82695]
- Salmenperä, L., J. Perheentupa, and M. Siimes. 1985. Exclusively breastfed healthy infants grow slower than reference infants. Pediatr. Res. 19:307-312. [PubMed: 3982892]
- Sampson, D.A., and G.R. Jansen. 1985. The effect of dietary protein quality and feeding level on milk secretion and mammary protein synthesis in the rat. J. Pediatr. Gastroenterol. Nutr. 4:274-283. [PubMed: 2580963]
- Schutz, Y., A. Lechtig, and R. Bradfield. 1980. Energy expenditures and food intakes of lactating women in Guatemala. Am. J. Clin. Nutr. 33:892-902. [PubMed: 7189091]
- Singh, J., A.M. Prentice, E. Diaz, W.A. Coward, J. Ashford, M. Sawyer, and R.G. Whitehead. 1989. Energy expenditure of Gambian women during peak agricultural activity measured by the doubly-labeled water method. Br. J. Nutr. 62:315-329. [PubMed: 2819016]
- Sosa, R., M. Klaus, and J.J. Urrutia. 1976. Feed the nursing mother, thereby the infant. J. Pediatr. 88:668-670. [PubMed: 943496]
- Strode, M.A., K.G. Dewey, and B. Lönnerdal. 1986. Effects of short-term caloric restriction on lactational performance of well-nourished women. Acta Paediatr. Scand. 75:222-229. [PubMed: 3754376]
- Stuff, J.E., and B.L. Nichols. 1989. Nutrient intake and growth performance of older infants fed human milk. J. Pediatr. 115:959-968. [PubMed: 2585235]
- Stuff, J.E., C. Garza, C. Boutte, J.K. Fraley, E.O. Smith, E.R. Klein, and B.L. Nichols. 1986. Sources of variance in milk and calorie intakes in breastfed infants: implications for lactation study design and interpretation. Am. J. Clin. Nutr. 43:361-366. [PubMed: 3953474]
- Terkel, J., C.A. Blake, V. Hoover, and C.H. Sawyer. 1973. Pup survival and prolactin levels in nicotine-treated lactating rats. Proc. Soc. Exp. Biol. Med. 143:1131-1135. [PubMed: 4743694]
- Tully, J., and K.G. Dewey. 1985. Private fears, global loss: a cross-cultural study of the insufficient milk syndrome. Med. Anthropol. 9:225-243. [PubMed: 3842148]
- Tyson, J.E., J.N. Carter, B. Andreassen, J. Huth, and B. Smith. 1978. Nursing-mediated prolactin and luteinizing hormone secretion during puerperal lactation. Fertil. Steril. 30:154-162. [PubMed: 680192]
- van Raaij, J.M.A., M.E.M. Peek, S.H. Vermaat-Miedema, C.M. Schonk, and J.G.A.J. Hautvast. 1987. Energy requirements of lactating Dutch women. Pp. 113-123 in Infant Growth and Nutrition. Proceedings of a Workshop. Foundation for the Advancement of the Knowledge of the Nutrition of Mother and Child in Developing Countries. Ede, The Netherlands.
- van Steenbergen, W.M., J.A. Kusin, and M.M. Van Rens. 1981. Lactation performance of Akamba mothers, Kenya. Breastfeeding behavior, breast milk yield and composition. J. Trop. Pediatr. 27:155-61.
- van Steenbergen, W.M., J.A. Kusin, C. de With, E. Lacko, and A.A.J. Jansen. 1983. Lactation performance of mothers with contrasting nutritional status in rural Kenya. Acta Paediatr. Scand. 72:805-810. [PubMed: 6673480]
- van Steenbergen, W.M., J.A. Kusin, S. Kardjati, and C. de With. 1989. Energy supplementation in the last trimester of pregnancy in East Java, Indonesia: effect on breast-milk output. Am. J. Clin. Nutr. 50:274-279. [PubMed: 2756913]
- Venkatachalam, P.S., and C. Gopalan. 1960. Basal metabolism and total body water in nursing women. Indian J. Med. Res. 48:507-510.
- WHO (World Health Organization) Task Force on Oral Contraceptives. 1988. Effects of hormonal contraceptives on breast milk composition and infant growth. Stud. Fam. Plann. 19:361-369. [PubMed: 2906764]
- Wagner, G., and A.R. Fuchs. 1968. Effect of ethanol on uterine activity during suckling in post-partum women. Acta Endocrinol. 58:133-141. [PubMed: 5695044]
- Wallgren, A. 1944/1945. Breast milk consumption of healthy full-term infants. Acta Paediatr. 32:778-790. [PubMed: 21007881]
- Warman, N.L., and K.M. Rasmussen. 1983. Effects of malnutrition during the reproductive cycle on nutritional status and lactational performance of rat dams. Nutr. Res. 3:527-545.
- Whichelow, M.J., and B.E. King. 1979. Breastfeeding and smoking. Arch. Dis. Child. 54:240-241. [PMC free article: PMC1545241] [PubMed: 434915]
- Whitehead, R.G., editor. ed. 1983. Maternal Diet, Breast-Feeding Capacity, and Lactational Infertility. Report of a joint UNU/WHO workshop held in Cambridge, United Kingdom, 9-11 March 1981. Food and Nutrition Bulletin, Suppl. 6. United Nations University, World Health Organization, Tokyo. 107 pp.
- Whitehead, R.G., and A.A. Paul. 1981. Infant growth and human milk requirements: a fresh approach. Lancet 2:161-163. [PubMed: 6114240]
- Wilde, C.J., C.V.P. Addey, M.J. Casey, D.R. Blatchford, and M. Peaker. 1988. Feedback inhibition of milk secretion: the effect of a fraction of goat milk on milk yield and composition. Q. J. Exp. Physiol. 73:391-397. [PubMed: 3399621]
- Winikoff, B., P. Semeraro, M. Zimmerman. 1988. Contraception During Breastfeeding—A Clinician's Source Book. The Population Council, New York. 36 pp.
- Wong, W.W., N.F. Butte, C. Garza, and P.D. Klein. 1990. Estimation of milk intake by deuterium dilution. Pp. 359-367 in S.A. Atkinson, editor; , L.A. Hanson, editor; , and R.K. Chandra, editor. , eds. Human Lactation 4: Breastfeeding, Nutrition, Infection and Infant Growth in Developed and Emerging Countries. ARTS Biomedical Publishers and Distributors, St John's, Newfoundland, Canada.
- Woolridge, M.W., and J.D. Baum. 1988. The regulation of human milk flow. Pp. 243-257 in B.S. Lindblad, editor. , ed. Perinatal Nutrition. Bristol-Myers Nutrition Symposia, Vol. 6. Academic Press, San Diego.
- Woolridge, M.W., N. Butte, K.G. Dewey, A.M. Ferris, C. Garza, and R.P. Keller. 1985. Methods for the measurement of milk volume intake of the breastfed infant. Pp. 5-21 in R.G. Jensen, editor; and M.C. Neville, editor. , eds. Human Lactation: Milk Components and Methodologies. Plenum Press, New York.
- Young, M.C., and K.M. Rasmussen. 1985. Effects of varying degrees of chronic dietary restriction in rat dams on reproductive and lactational performance and body composition in dams and their pups. Am. J. Clin. Nutr. 41:979-987. [PubMed: 3993613]
- Zuppa, A.A., A. Tornesello, P. Papacci, G. Tortorolo, G. Segni, G. Lafuenti, E. Moneta, A. Diodato, M. Sorcini, and S. Carta. 1988. Relationship between maternal parity, basal prolactin levels and neonatal breast milk intake. Biol. Neonate 53:144-147. [PubMed: 3370259]
Footnotes
- 1
Mean, 1 kg (˜ 2 lb); range, 0 to 6 kg (˜ 13 lb).
- 2
Mean total energy intake was 2,739 kcal/day for the exercising subjects compared with 2,051 kcal/day for the sedentary controls.
- Milk Volume - Nutrition During LactationMilk Volume - Nutrition During Lactation
Your browsing activity is empty.
Activity recording is turned off.
See more...
