Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Julious SA, Horspool MJ, Davis S, et al. PLEASANT: Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term – a cluster randomised controlled trial and economic evaluation. Southampton (UK): NIHR Journals Library; 2016 Dec. (Health Technology Assessment, No. 20.93.)

PLEASANT: Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term – a cluster randomised controlled trial and economic evaluation.
Show detailsThis review aimed to identify preference-based utility values for asthma day-to-day symptoms (baseline utility) and asthma exacerbation.
Scoping
A scoping search was conducted to establish the likely quantity and relevance of published literature. This was done by searching MEDLINE and The Cochrane Library (Cochrane Database of Systematic Review, HTA database and NHS Economic Evaluation Database) using a limited number of population terms in addition to a search filter for quality of life. It was found that there was a lack of utility data derived from EQ-5D in children with asthma. Although EQ-5D is the preferred outcome measure, the standard version of EQ-5D is not designed to be used with children. EQ-5D-Youth is available for children and adolescents, but there is not yet a validated UK tariff. In view of this, the NICE reference case states to use other validated preference-based measures developed for children, but does not specify the preferred quality-of-life instrument.35 Therefore, a broad approach was taken in the search to identify utility values derived from EQ-5D, as well as other preference-based measures. EQ-5D values estimated from mapping studies were also considered.
Search strategy
Search terms
Both free text and medical subject headings (MeSH) pertaining to children, asthma and asthma exacerbation were used in the search (see Appendix 6). The InterTASC Information Specialists’ Sub-Group (ISSG) search filter was used to filter studies that report HRQoL (see Appendix 7). The filter was adapted to include a newly developed preference-based measure for children, Child Health Utility Index 9D,44 as well as other preference-based measures in asthmatic children, such as Asthma Symptom Utility Index. Full search terms for this review are presented Appendices 6 and 7.
Search limit
The search was not limited by language, publication type, publication dates or study design, with the aim of increasing sensitivity.
Sources searched
The following clinical and economic databases were searched:
- MEDLINE (via Ovid) (In-Process & Other Non-Indexed Citations) (1946 to 5 July 2014)
- The Cochrane Library (includes Cochrane Database of Systematic Review, NHS Economic Evaluation Database and HTA database) (up to 5 July 2014)
- EMBASE (1974 to 5 July 2014)
- EconLit (1886 to 5 July 2014)
- School of Health and Related Research (ScHARR) Health Utilities Database (up to 5 July 2014).
In addition to the electronic database search, reference lists of the retrieved papers were screened for relevant papers.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the review are summarised in Table 30. Systematic reviews and protocols were not included, but were used to identify relevant papers. Modelling studies were examined to determine the source of utility values used. Modelling studies that described utility data not reported elsewhere were included in the review.
TABLE 30
Review inclusion and exclusion criteria
Selection of studies
In the first stage of study selection, titles and abstracts of the searched results were screened against the inclusion/exclusion criteria. Full articles were assessed if titles and abstracts were unclear. All studies identified at titles and abstracts were further screened at full text. The studies were screened by a single reviewer.
Quality assessment
Quality assessment of articles in this review followed the criteria (sample size, number loss at follow-up and handling of missing data) recommended by Papaioannou et al.45 in the Decision Support Unit Technical Support Document on the identification, review and synthesis of health state utility values from the literature.
Data extraction
Data extracted comprised characteristics of study population, study design and details of outcome measurements (descriptive system, tariff used, method of valuation, time of measurement, mean utility data and other relevant measures).
Selection of utility data for use in the economic analysis
Selection of utility data to use in the economic analysis was based on (1) quality of the study; (2) the relevance of utility data to the population and health states in the PLEASANT study; and (3) the extent to which the measurement method was in accordance with the NICE reference case.
Results of systematic review of health-related quality-of-life data
A total of 927 studies were retrieved from the database search and reference tracking. After removal of duplicates, 683 studies were screened at titles and abstract. A total of 659 studies were excluded at this stage (see Appendix 8). Subsequently, 24 papers were screened at full text and 10 papers were excluded, with reasons given in Appendix 9. Finally, 14 papers were included in this review. Figure 27 shows the search process of this review.
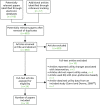
FIGURE 27
Flow diagram of search process. EQ-VAS, EuroQol Visual Analogue Scale.
Study characteristics for the included studies are summarised in Table 31. The study populations are summarised in Table 32 and methods used to measure HRQoL are summarised in Table 33. Details regarding study quality are provided in Table 34. Details regarding the suitability of the studies for use in the economic model, based on the criteria described above, are provided in Table 35.
TABLE 31
Characteristics of included studies
TABLE 32
Population of included studies
TABLE 33
Outcome measurement and utility values in each study
TABLE 34
Quality assessments of included papers
TABLE 35
Relevance of studies to the PLEASANT study analysis and the NICE reference case
Six studies included UK patients,38,46,47,52,53,56 three of which were multinational studies.38,53,56 Three papers were from the USA,49,55,57 two were based in Canada50,51 and one each was from the Netherlands,36 Belgium48 and Spain.54 Only the studies by Juniper et al.,51 Chiou et al.49 and Powell et al.46 directly measured HRQoL in populations confined to children. Chiou et al.49 recruited children aged between 7 and 12 years with diagnosed asthma of at least mild persistent severity, while Juniper et al.51 studied children with symptomatic asthma with a mean age of 12 years (range 7–17 years) and Powell et al.46 included children aged between 2 and 16 years with acute severe asthma. Two studies, by Rodríguez-Martínez et al.54 and Carroll and Downs,55 elicited preferences from parents regarding health states in children. Other studies comprised populations with mixed age groups. Of these, the studies by Mittmann et al.50 and Willems et al.36 presented HRQoL data stratified by age.
The populations in the included studies differed in asthma severity and characteristics. Five studies measured HRQoL using EQ-5D.36,46–48,52 Other studies used outcome measurements, such as the Pediatric Asthma Health Outcome Measure (PAHOM) (n = 2) and Health Utilities Index Mark 2 (HUI-2) (n = 1) and Mark 3 (HUI-3) (n = 1). Direct valuation using vignettes was used in two studies. This review also included three modelling studies,38,53,56 which estimated EQ-5D data from mapping exercises.
The EQ-5D is a generic preference-based measure in which the descriptive systems consist of five dimensions: mobility, depression/anxiety, self-care, usual activities, pain and discomfort. Each dimension has three levels of severity, and this gives rise to 243 possible health states described by the EQ-5D. In the UK, scoring of EQ-5D was based on time trade-off (TTO) in a representative sample of 2997 adults administered using York Measurement and Valuation of Health TTO protocol. Public preferences were obtained for 43 health states and regression was used to model data for the remaining health states. Utility score from the algorithm was anchored at ‘1’ for perfect health and ‘0’ for a state equivalent to death.58
Willems et al.,36 Price et al.47 and Powell et al.46 were randomised controlled trials (RCTs) that elicited an EQ-5D index score using UK preferences, whereas the EQ-5D score in a cohort study by Brusselle et al.48 was based on the Belgian tariff. Norman et al.52 was a modelling study that used EQ-5D data collected from the Evaluate Xolair for Asthma as Leading Treatment (EXALT) trial. The tariff used in the EXALT study is not described by Norman et al.,52 but the data are described as being consistent with the NICE reference case, suggesting that the UK TTO valuation set was used.
In the MAGNEsium Trial In Children (MAGNETIC), Powell et al.46 included a population of children (n = 508) with severe acute exacerbations, as defined by British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN). The MAGNETIC was a prospective, double-blind, multicentre RCT in the UK, designed to compare efficacy of nebulised magnesium sulphate with usual care. EQ-5D and Paediatric Quality of Life Inventory (PedsQL™) postal questionnaires were collected at 1 month post exacerbation. EQ-5D data were obtained for children aged ≥ 5 years and were filled out by parents as proxy, while PedsQL™ were obtained for all children and were self-completed if children were over 5 years. Respondents were asked to recall events in the previous 4 weeks while filling out the outcome measures. The adult UK tariff was applied to EQ-5D to obtain utility value for each child. Utility values for patients under 5 years were estimated through mapping between EQ-5D and PedsQL™. In this study, baseline EQ-5D data during exacerbation were not collected for ethical reasons. Therefore, asthma symptom scores (ASSs) at exacerbation were mapped to EQ-5D based on experts’ opinions. The expert team comprised a paediatric consultant and two respiratory nurses who routinely treated asthmatic paediatric patients. An EQ-5D health state of 11111 was assigned to ASSs of 1–3 in the base case, while ASSs of 4–6 and 7–9 were mapped to EQ-5D health states of 22222 and 33333, respectively. In our opinion, the subjective nature of this mapping between ASS and EQ-5D was considered to make the EQ-5D scores estimated at the time of exacerbation very uncertain. Furthermore, these data would be relevant only to the subgroup of patients who have severe acute exacerbations requiring treatment in secondary care, as this was the population recruited into the MAGNETIC. This study was blinded to patients, health-care providers and outcome analysts. Therefore, it had low risk of performance and detection bias. However, the study was subjected to risk of attrition bias due to the low response rate of EQ-5D questionnaires. The authors addressed this limitation by using a mapping function to estimate EQ-5D data for those with PedsQL™ data. This was based on the subset of patients for whom both PedQL and EQ-5D data were available. Following mapping estimations, a total of 218 EQ-5D data were available for analysis for the outcome 1 month after exacerbation.
Price et al.47 included patients in the UK aged between 12 and 80 years with poorly controlled asthma at BTS/SIGN treatment step 2 or 3. The mean age of patients was 44.74 years (SD 16.49 years) at step 2 and 50.02 years (SD 15.93 years) at step 3. In step 2 patients, a leukotriene receptor antagonist was compared with inhaled corticosteroid. In step 3 patients who were already receiving inhaled corticosteroid, a leukotriene receptor antagonist was compared with a long-acting β2-agonist. EQ-5D data were directly measured from patients and were presented by treatment steps and interventions at baseline, 2 months and 2 years. Utility values were estimated using UK preferences. This RCT had a high retention rate, with 5–10% loss to follow-up. A large proportion (75%) of patients presented with less than four missing data, and missing data were handled using multiple imputation. This single-blind RCT (n = 687) was robust, with large sample size, low risk of attrition bias and measured outcomes with EQ-5D. However, utility data presented were not stratified by age nor related to asthma exacerbations. Therefore, these data lack applicability to the PLEASANT trial and the health states modelled.
Willems et al.36 used UK preferences to estimate utility scores for asthmatic patients in the Netherlands. Populations comprised adults (n = 53) and children (n = 56) with mild to moderate asthma [Global Initiative for Asthma (GINA) states I–III]. EQ-5D questionnaires were filled by carers for children under 12 years and self-completed for those aged ≥ 12 years. There were only four children lost to follow-up, and various imputation techniques were applied. Missing baseline scores were imputed with mean scores. Quality of life scores at baseline (usual care 0.96, nurse monitoring 0.92) were consistent with the good lung function of the study’s population [mean forced expiratory volume in the first second (FEV1) above 90% predicted]. However, these results were elicited from a non-UK population, but did use a UK valuation set. Willems et al.36 did not examine the utility decrement in exacerbation.
Brusselle et al.48 conducted a 1-year cohort study (n = 158) to determine the efficacy and safety of omalizumab by looking at changes from baseline in a single-arm study. The mean age of the population studied was 48.17 years (SD 17.18 years) and age ranged from 12 to 83 years. Patients included had poorly controlled severe allergic asthma (FEV1 < 80% predicted) and past history of exacerbations. The Belgian tariff was applied to the collected EQ-5D data at baseline and 1 year. Only 126 of 158 patients had baseline EQ-5D values, and only 67 had EQ-5D data at 1 year. Handling of missing data, however, was not reported. This tariff was obtained from public preferences in Belgium using the visual analogue scale (VAS) valuation method.59 However, valuation using VAS is not a choice-based method. In the UK, NICE expressed a preference of using TTO as the valuation method, and, in the absence of TTO, other choice-based methods such as subgroup analysis are preferred over VAS.60 Therefore, utility data estimated from this study do not meet the NICE requirement of using a choice-based valuation method.
Two USA-based studies, by Chiou et al.49 and Gerald et al.,57 used PAHOM, an asthma-specific preference-based measure designed for children. It consists of a descriptive system with three dimensions: symptoms, emotions and activity. The symptoms dimension is classified to three levels of severity, while emotions and activity are dichotomous choices to indicate presence or absence of problems. Unlike EQ-5D with a recall period of 1 day, respondents are asked to describe health states for the past 7 days using PAHOM. The utility value of a health state is calculated as the average utility values over 7 days. Preference weights for PAHOM were elicited from 114 adults in Seattle, WA, USA, who responded for children. Subgroup analysis and VAS were used to value health states. As not all health states were valued using subgroup analysis, because of cognitive burden, VAS values were transformed into subgroup analysis values using relative risk attitude equation.49
Chiou et al.49 measured utility value in 72 children (aged 7–12 years) with diagnosed asthma of at least mild persistent severity as 0.83 (converted subgroup analysis value). Chiou et al.49 also reported mean VAS and subgroup analysis values for patients according to asthma severity, with subgroup analysis values of 0.79 for mild or no symptoms, 0.70 for moderate and 0.28 for severe. A limitation of this study was the small sample size, which may have affected the accuracy and validity of results, particularly for the estimates stratified by severity. Values stratified by presence or absence of exacerbation were not reported.
Gerald et al.57 performed a modelling study on different screening strategies for asthma. A decision tree and Markov models for a cohort of children were constructed. The Markov model consists of five health states: asthma symptom-free day, symptom days, exacerbation recovery days, emergency department visits and hospitalisation days. The utility value for each health state was derived using PAHOM. PAHOM states were allocated to the modelled health states. When several PAHOM states could describe a modelled health state, utility values of the relevant states were averaged to estimate a single utility value. For example, three or four PAHOM states were thought to characterise ‘symptom days’ in the model. The utility values of these states were averaged to derive utility value for ‘symptom days’. The authors highlighted that this approach may fail to capture valuation of ‘symptom days’ accurately. In our opinion, the subjective nature of this mapping from modelled health states to PAHOM states reduces the robustness of these utility estimates. In addition, a general concern regarding PAHOM was that this measure was not validated for its psychometric properties. Furthermore, validation of the relative risk attitude equation used to derive subgroup analysis values was not performed.49
Two Canadian-based observational studies used the Health Utilities Index (HUI) as an outcome measure. Juniper et al.51 studied the minimum skills required by children to complete outcome measurements unassisted. The Paediatric Asthma Quality of Life Questionnaire, Feeling Thermometer, HUI and direct valuation were administered to 52 children aged 7–17 years (mean 12 years) with symptomatic asthma (mean FEV1 85% predicted). The HUI-2 Canadian tariff was applied to obtain utility value. The mean HUI baseline value for asthma was reported as 0.89 (SD 0.09).
The six-dimensional version of HUI-2 is a common generic outcome measure in children. Each dimension has 3–5 levels, allowing 8000 unique health states to be defined. The HUI-2 tariff was estimated from a sample of 293 parents of school children in Ontario, Canada. Valuations were performed using VAS and three health states were valued with VAS and subgroup analysis. A power function was then derived to map VAS values to subgroup analysis values, and multiattribute utility theory was used to derive the valuation functions.58
Mittmann et al.50 conducted a cross-sectional study to measure HRQoL of 20 chronic diseases. The HUI-3 was administered through interview to 17,626 household residents (≥ 12 years) in Canada. HUI-3 is an adapted version of HUI-2 with additional dimensions and levels. HUI-3 weights were elicited from a random sample of adults (n = 504) in Ontario, Canada. In this study, however, the HUI-2 scoring algorithm was used for HUI-3 data. The mean HUI score reported for children (aged 12–19 years) with asthma was similar to that reported by Juniper et al.51
In measuring and valuing children’s health, NICE is less clear on the preferred instrument, but advises use of a standardised and validated preference-based measure designed for children. Although HUI is an example of an instrument that meets the mentioned criteria, the HUI data from these studies may not be valid, as the study designs lack rigour. First, the small sample size (n = 52) recruited by Juniper et al.51 may introduce inaccuracy to the results. Second, HUI-3 data were inappropriately scored in the study by Mittmann et al.50 and utility scores estimated were deemed to be provisional by the authors. Furthermore, neither of these studies reported the utility decrement attributable to asthma exacerbation.
Four modelling studies performed mapping to estimate EQ-5D values. Brown et al.56 and Norman et al.52 constructed Markov models to evaluate the cost-effectiveness of omalizumab in addition to standard care. Norman et al.52 used EQ-5D scores measured in the EXALT study for day-to-day asthma symptoms. The EXALT study was an open-label RCT, comprising 404 patients in the UK (age range from 12 to 75 years) with poorly controlled severe allergic asthma (FEV1< 80% predicted). Utility for day-to-day symptoms (by treatment arm) was estimated from EQ-5D scores recorded in the EXALT study.
Norman et al.52 also conducted a systematic review of HRQoL literature to identify HRQoL data of relevance to both adult and paediatric populations. In their base-case analysis they used data from Lloyd et al.,37 a study conducted in an adult population that provides estimates of the health utility decrement (loss) associated with exacerbations requiring oral steroid treatment and exacerbations requiring hospitalisation. The decrement was measured by comparing baseline EQ-5D values with those reported at 4 weeks for patients who did and did not experience exacerbations during that 4-week period. They cited another study by Steuten et al.,61 which also provided utility values for exacerbations in an adult population. However, this study collected data at 3- to 6-month intervals, which could make it harder to detect the relationship between short-term exacerbations and health utility than the 4-week interval used by Lloyd et al.37
Brown et al.56 used a published algorithm by Tsuchiya et al.62 to map the mini-Asthma Quality of Life Questionnaire (AQLQ) scores from the ETOPA trial onto the EQ-5D. The ETOPA trial was a multinational open-label trial that recruited 312 patients aged between 12 and 73 years (mean > 35 years) with poorly controlled allergic asthma (mean FEV1 < 73% predicted).63 (Note that Brown et al.56 used data from the subgroup of ETOPA patients with severe disease, but baseline characteristics are not described for this subgroup, so Table 32 provides characteristics for the ETOPA trial as a whole.) The AQLQ scores were mapped to EQ-5D for patients separated by disease state and responder status. The mapping algorithm used by Brown et al.56 was derived from a RCT of 3000 adults in the UK with a wide range of asthma.62 In the RCT used to generate the mapping algorithm, both EQ-5D and AQLQ were collected.64 Domains in EQ-5D were found to overlap with those in AQLQ, with correlations between 0.56 and 0.65. Six main mapping models and two supplementary models were derived using the regression method and were validated using an external data set. However, these mapping functions were associated with large marginal errors, and should be considered only as second best to direct elicitation of EQ-5D data.58
In the economic modelling study by Brown et al.,56 literature-based estimates were used to model the decrement associated with exacerbations, as the authors stated that the ETOPA trial collected insufficient patient quality-of-life data during exacerbations. The literature-based estimates cited by Brown et al.56 appear to be from an earlier publication65 of the study by Lloyd et al.37
The modelling studies by Briggs et al.38 and Doull et al.53 mapped AQLQ scores from the 52-week Gaining Optimal Asthma ControL (GOAL) trial onto EQ-5D values. The GOAL study was a multinational double-blind RCT designed to evaluate efficacy of a combination of fluticasone/salmeterol compared with fluticasone in terms of asthma control. The GOAL study comprised 3416 patients (mean age > 35 years; range 12–80 years) with uncontrolled asthma (mean FEV1 < 80% predicted) from 44 countries.66 Asthma control in the GOAL trial was classified as totally controlled, well controlled, not well controlled or exacerbation requiring oral steroid or secondary care by Briggs et al.38 using the GINA definition. As the GOAL trial collected only AQLQ data, a mapping function obtained through personal communication with Macran and Kind (no further details of this communication provided by Briggs et al.38) was used to transform AQLQ scores to EQ-5D values. Subsequently, the utility value for each asthma control health state was derived using regression. In the regression model, a UK indicator was added as a dummy variable to adjust for a UK specific-population. The dependent variable was the utility value, whereas asthma control and the UK indicator were the independent variables. Both independent variables were found to be significant predictors of quality of life. The quality-of-life data from this study are of relevance to the PLEASANT trial. However, the mapping function used in the analysis by Briggs et al.38 was inadequately described by the authors, and a published article providing more details could not be identified from searches. Therefore, an assessment of mapping performance was not possible.
Doull et al.53 adapted the analysis by Briggs et al.,38 and reclassified asthma control to ‘symptom free’ and ‘with symptoms’. Totally controlled asthma was classified as ‘symptom free’, while other states were classified as ‘with symptoms’. The weekly utility in the ‘with symptom’ state was equivalent to the weighted average of the weekly utility in well controlled, not well controlled and exacerbation health states from Briggs et al.38 Regression was used to estimate the relationship between asthma control and quality of life, when quality of life was obtained by mapping AQLQ scores to EQ-5D. Asthma control and the UK indicator were entered into the model as the independent variables, while weekly utility was entered as the dependent variable. Subsequently, utility for the ‘with symptoms’ and the ‘symptoms-free’ health states were estimated from the regression coefficients. As utility data in this study were adapted from Briggs et al.,38 which mapped AQLQ scores to EQ-5D using the mapping function by Macran and Kind, the validity of mapped data was likewise not assessable.
The method used in Carroll and Downs55 and Rodríguez-Martínez et al.54 involved valuation of hypothetical health states by parents. Parents were asked to value health states described in vignettes by imagining their children affected by those states. Descriptions in vignettes, however, differed across studies. Rodríguez-Martínez et al.54 developed asthma-specific vignettes based on PAHOM,49 and these were validated by expert opinions, whereas Carroll and Downs55 developed general descriptions of 29 health states with the inclusion of time as a factor. Rodriguez-Martinez et al.54 requested parents (n = 76) to value vignettes using subgroup analysis, while Carroll and Downs55 used subgroup analysis and TTO methods in a sample of 4016 parents (Note that each parent valued only three of a potential 29 states providing around 415 values per state.) Neither study constructed vignettes based on rigorous methods such as a focus group. The lack of standardised descriptive systems of vignettes and different valuation methods also resulted in a lack of comparability of results between studies. In addition, vignettes are limited to specific descriptions of a condition, and may not fully reflect all experiences of a patient. Therefore, vignettes do not meet the NICE reference case and are considered of little value in economic evaluation.60 In view of the various limitations associated with vignettes, utility values from Carroll and Downs55 and Rodríguez-Martínez et al.54 were not considered suitable for use in the PLEASANT study economic analysis.
Health state utility values used in the analysis
The utility values used in the economic evaluation by Briggs et al.38 appear to be particularly relevant to our proposed model structure, as they are reported for relevant health states, including an exacerbation state, and have been estimated from a trial population that included some children. However, the mapping algorithm used to convert from the condition-specific HRQoL measure (AQLQ) to the EQ-5D utility score is not from a published source, and is not described in detail, making it difficult to assess its validity. However, if the values reported by Briggs et al.38 are taken at face value, they provide an estimate of the utility loss for exacerbation versus total asthma control of –0.216 (SE 0.007). It is possible that some patients do not have total asthma control in the absence of an exacerbation and the difference between the utility values for the exacerbation state and the not well controlled states is smaller, at –0.112. The data from Briggs et al.38 suggest that the utility decrement for exacerbation in the average patient is likely to fall in the range –0.112 to – 0.216. The utility decrements provided by Lloyd et al.37 from an adult population are –0.1 and –0.2 for exacerbations requiring oral steroids and exacerbations requiring hospitalisation, respectively. It therefore appears that there is reasonable agreement between the values reported by Briggs et al.38 and Lloyd et al.37
We accept that the estimates provided by Briggs et al.38 and Lloyd et al.37 probably underestimate the degree of utility loss in children with a severe or life-threatening acute exacerbation during the period of hospitalisation. This is because the utility values were not measured during the acute exacerbation period itself. In the MAGNETIC, which estimated utility scores in children attending EDs with severe acute asthma, the utility was estimated to be reduced from a baseline of 0.88 to 0.516 during the initial acute period, giving a utility decrement of 0.364. However, in the MAGNETIC, this more severe utility decrement was only applied until hospital discharge, with the average length of hospital stay being 1 day. If we apply a decrement of 0.364 for 1 day and assume a loss of 0.2 in the remaining 6 days, the average utility loss over the whole week of exacerbation (–0.22) would be similar to that reported by Briggs et al.38
Given the uncertainty regarding the mapping algorithm used by Briggs et al.,38 and the previous use of data from Lloyd et al.37 in a number of published economic evaluations, we decided to use the data from Lloyd et al.37 in the base-case analysis. The data from Briggs et al.38 have been explored in a sensitivity analysis using the difference between the total control state and the exacerbation state (–0.216) to estimate the quality-of-life decrement from exacerbations. This sensitivity analysis is considered to provide an upper limit on the utility decrement attributable to exacerbation.
For patients without an exacerbation, we have taken the baseline utility score for the control arm of the study by Willems et al.,36 as this provides an estimate based on the child version of the EQ-5D valued using the adult UK TTO valuation set. The population was Dutch children aged 7–18 years with a GINA severity stage I–III receiving standard outpatient care. The value applied to patients without an exacerbation will affect the calculation of absolute QALYs in each trial arm, but does not affect the estimation of incremental QALY gain that goes into the cost-effectiveness ratio. Therefore, the selection of this data source is less critical than that used to determine the decrement attributable to exacerbations. The data that have been applied in the model are summarised in Health outcomes.
- Systematic review of health-related quality of life data to inform health econom...Systematic review of health-related quality of life data to inform health economic analysis - PLEASANT: Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term – a cluster randomised controlled trial and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...