The 'Copy' Feature
The 'copy' feature enables submitters to seed a new test submission by duplicating the information from another test that has already been submitted.
Making the copy feature work for you
Specify a new, unique test name to clearly distinguish the copied test from the original. Delete or edit information that is automatically copied to the new test if it is specific to the original test. Ensure tests are not exact duplicates of each other by carefully reviewing the copied fields such as condition names, test targets and methodologies.
Examples where the copy feature may be useful:
- A submitter enters test information for a sequencing test for the L1CAM gene and submits the test. The submitter uses the copy feature to create a new test submission. A new, unique test name is entered. The test method is changed to duplication/deletion analysis. The analytical validity statement is revised. The time required to register the second test is reduced.
- A submitter enters and submits a panel test that assesses 5 genes by sequence analysis. The laboratory also offers 5 tests, each of which evaluates one gene at a time. The submitter copies the panel test 5 times. Within each of the 5 copied tests, the test target is changed to represent only the single gene being assessed (the other 4 are deleted). Other relevant fields are edited (as appropriate) to reflect each specific test, such as test name, condition name, analytical validity statement, methodology, order code, etc. This allows for efficient entry of 6 tests in total.
Availability of copy feature
- Available only for submitted tests. Test that are in Edit mode cannot be copied.
- Available for both clinical and research tests.
- Clinical and research tests can be used to seed the same type of test but cannot be used to seed a different type of test (clinical tests cannot seed research tests, or vice-versa).
List of fields that are NOT copied into a new test registration page:
Clinical Tests
Laboratory Test Name
Short Test Name
Manufacturer's Test Name
Test Order Code
Default parameters
Research Tests
Laboratory Test Name
Short Test Name
How to Copy a Test
Log into the laboratory's submission homepage (See information on accessing and logging into the GTR submission site.
1. Go to the GTR Submssion home page
In the section 'Tests in this lab', click on 'Manage tests' to go to the Test management page.
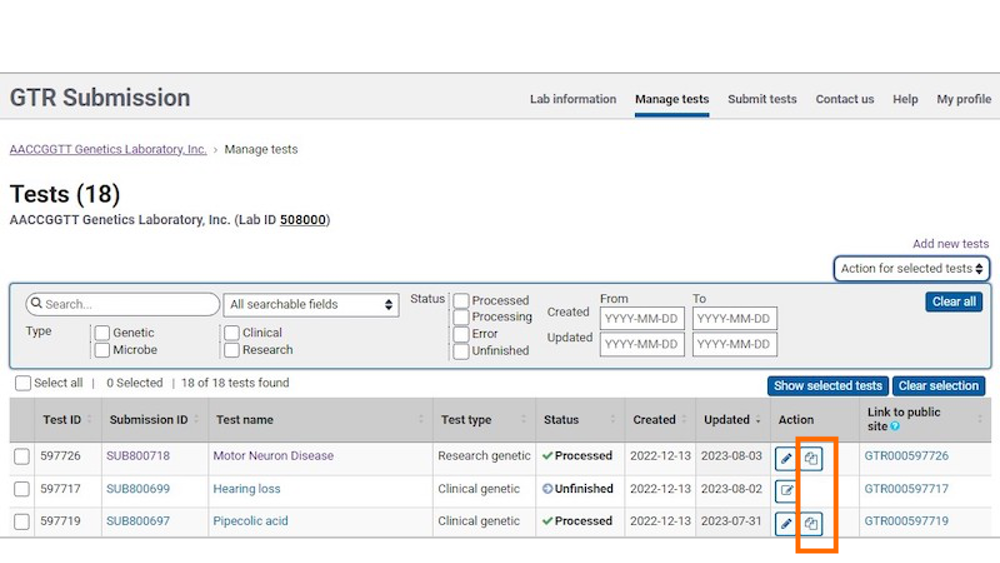
2. Copy the test
In the table, locate the test you want to copy. In the Action column, you will locate the copy icon. Click the copy icon to copy the test.
To copy data from a submitted test into a new test registration, click the 'copy' link.
3. Complete the copied test
After clicking the 'copy' link, you will be taken to the Basics tab of the newly copied test. Data from the original test will automatically be populated in this new test, with the exception of the fields listed above. All fields in the new test can be edited, including those that were copied. Pay special attention to fields that may be specific to the original test and either edit or delete the information.
NOTE: At a minimum, the 'Laboratory Test Name' field must be completed in order to submit the new copied test.
4. Submit the test
We highly recommend you review all tabs before submitting your copied test.
Tests are not processed until the 'Submit' button is clicked on the test 'Review & Submit' tab.
Once the copied test is submitted, it can be copied as well.
