NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Duarte A, Mebrahtu T, Goncalves PS, et al. Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2017 Nov. (Health Technology Assessment, No. 21.64.)

Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation.
Show detailsIntroduction
The review of cost-effectiveness evidence in the population of children and young people and the absence of company models highlights the challenges of developing an economic model in this population. The fundamental challenge is the limited clinical evidence base for both short- and long-term outcomes to inform a model. Therefore, any estimation of the cost-effectiveness of biological therapies in children and young people will be subject to a number of uncertainties. These uncertainties cannot be avoided but a clear and transparent approach, that highlights the assumptions entering the economic model, can be pursued to help the decision-maker assess the cost-effectiveness of biological therapies in this population.
Plaque psoriasis is a chronic non-progressive disease that manifests itself in children and young people in a similar manner to that in adults. The main difference between the younger population and adults is the presence of comorbidities in adults (such as high blood pressure, liver impairment and renal impairment), which tend to make adults less well with psoriasis than a younger population. Currently, there is no treatment pathway specific to psoriasis in children and young people in the UK. The management of treatment and approach to care seems to mirror that used in adults. Our clinical advisor, Dr Ruth Murphy, indicated that when there is an absence of evidence it would be reasonable to extrapolate data from the adult population to children and young people. The company submission for ustekinumab also supports this approach for the development of an economic model given that there are few significant differences in the posology or management of chronic plaque psoriasis in children, young people and adults.
The management and treatment of plaque psoriasis depends on the extent and severity of an individual’s disease, local custom and practice. If an individual patient does not respond to or tolerate a particular treatment option, an alternative one is usually tried. This means that treatments are usually ‘trialled’ on an individual basis until an effective option is found. If an effective treatment is not found, then a patient will receive some form of BSC. This approach to treatment appears to be the same for children and young people and adults, but usually more caution is exercised in the younger population because of the limited availability of licensed treatment options.
The trialling of treatments on treatment failure or intolerance suggests that sequences of treatments could be considered in an cost-effectiveness model, whereby after failure of a first treatment option patients are trialled on a second option and so on, until all options are exhausted. However, this would require additional clinical evidence on the efficacy of the treatments conditional on the previous therapies received. There is very limited evidence to support the cost-effectiveness of the sequential use of treatments in adults and no evidence exists in children and young people (see Chapter 3). Therefore, although the model should ideally explore the sequential use of treatments, any attempt to do so in the population of children and young people would be highly uncertain. Furthermore, the optimum treatment sequence may not be suitable for an individual patient with specific characteristics and when treatment in this population is usually tailored to the child or adolescent because of needle phobia or the presence of psoriatic arthritis. Therefore, an alternative approach may be better whereby the optimum ordering of treatments, in terms of their cost-effectiveness, is established. This can be achieved by comparing each of the alternative treatment options with BSC and then indicating the most cost-effective order in which to give the therapies based on total expected costs and QALYs associated with each treatment option.
The previous York model appears to be the most widely accepted model of chronic plaque psoriasis.97 The five NICE TAs that followed TA103 for the treatment of moderate to severe psoriasis in adults98–102 followed the framework of the York model and these have been accepted by NICE as being relevant to plaque psoriasis. The main changes that have followed since the advent of the York model have been the availability of new evidence, the methodology for linking efficacy estimates to HRQoL utility values, the parameters used in the model to inform BSC, updated unit costs, time on treatment and the modelling of treatment sequences in the most recent appraisal of apremilast.102 It would therefore seem appropriate that the same modelling framework is used for children and young people but with an evidence base informed by outcomes in the younger population. Hence, the structure of our model is very similar to that used in previous TAs in adults and, when evidence is lacking or limited in the population of children and young people, data have been extrapolated from the adult population and supplemented by expert opinion.
Decision problem and patient population
The decision problem addresses the cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. The population in the model reflects the marketing authorisations of the three interventions. However, the marketing authorisations differ in terms of the age of the population and the severity of psoriasis at baseline and also in terms of the positioning of the biologic in the pathway of care.
A stepwise approach to treatment for the management of plaque psoriasis is usually pursued in which topical therapies are offered as first-line treatment followed by phototherapies and/or systemic non-biological therapies such as methotrexate as second-line treatment and then biological treatments as third-line treatment when previous therapies have been found to be ineffective. However, adalimumab is licensed in a paediatric population for individuals who have an inadequate response to, or who are inappropriate candidates for, topical therapy and phototherapies, whereas etanercept and ustekinumab are licensed for individuals who are inadequately controlled by, or who are intolerant to, other systemic therapies or phototherapies. Therefore, adalimumab is the only biological treatment indicated in the population of children and young people who have not failed previous systemic therapies.
The biological interventions also differ in their marketing authorisations by age and severity of psoriasis (Figure 9). Both adalimumab (age ≥ 4 years) and etanercept (age ≥ 6 years) are indicated for younger ages and severe psoriasis, whereas ustekinumab is indicated for an adolescent population (age ≥ 12 years) and moderate to severe psoriasis. The definition of severity differs in the corresponding trials of the biologics in children and young people (Table 56). In adults, severe psoriasis is defined by a total PASI score of ≥ 10 and a DLQI score of > 10. However, there is not a clear consensus on the definition of moderate or severe psoriasis in children and young people. This is partly because the PASI has not been validated as a disease severity assessment tool for use in this population and no other tool is available. Mean PASI scores at baseline in the trials were 18.6 for etanercept, 18.3 for adalimumab and 21.1 for ustekinumab. Therefore, although the licence for ustekinumab includes those with moderate to severe psoriasis, patients in the ustekinumab trial (CADMUS) had a disease severity that was more comparable to that in patients with severe disease in the etanercept trial (20030211) and adalimumab trial (M04-717). Hence, the population in the model was chosen to reflect severe psoriasis as defined by the baseline characteristics of the populations in the trials of children and young people.

FIGURE 9
Marketing authorisations of biological therapies in children and young people by age and severity.
TABLE 56
Definition of disease severity in the trial populations in trials of children and young people
To reflect the differences in marketing authorisation by age and positioning of treatment in the pathway, three separate populations were considered in the base-case cost-effectiveness analysis:
- Before systemic therapy – children and young people aged 4–17 years with adalimumab as the only licensed intervention for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies, that is, as an alternative to systemic therapies.
- After systemic therapy (1) – children and young people aged 6–11 years with adalimumab and etanercept for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
- After systemic therapy (2) – children and young people aged 12–17 years with adalimumab, etanercept and ustekinumab for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
The population aged 4–5 years with adalimumab as the only licensed intervention for the treatment of severe plaque psoriasis after systemic therapy was not considered as a separate population because no children aged < 6 years were included in the adalimumab trial (M04-717); therefore, efficacy estimates for this age group were assumed to be the same as those for children aged 6–11 years, which results in similar cost-effectiveness estimates for adalimumab compared with BSC for ages 6–11 years.
The starting age used in the model was 4 years, 6 years and 12 years for the three populations described above respectively. The time horizon of the model extended until individuals reached 18 years of age. At this point, the population reaches adulthood and separate NICE recommendations for the use of the interventions in adults apply. The differences in the marketing authorisations of the interventions by age inevitably mean that the time horizon of the model differs according to the population. To explore the impact of the time horizon, a separate scenario analysis is presented that considers a common time horizon of 14 years for all populations. The time horizon of 14 years (which is greater than the time horizon of 10 years used in previous TAs in adults) is sufficient to capture differences in costs and effects between the interventions under comparison.
Intervention and comparators
The interventions considered in the cost-effectiveness analysis were adalimumab, etanercept and ustekinumab within their marketing authorisations. The following comparators were considered in the NICE scope:147
- Non-biological systemic therapy (including, but not limited to, ciclosporin and methotrexate).
- Topical therapy (for people in whom non-biological systemic therapy is not suitable), that is, BSC.
- Biological treatments used outside their marketing authorisation (such as infliximab, adalimumab, etanercept or ustekinumab if used outside the constraints of the relevant marketing authorisation in children and young people).
- When appropriate, adalimumab, etanercept and ustekinumab will be compared with each other.
Because of the positioning of adalimumab in the stepwise management of psoriasis, non-biological systemic therapy is only a relevant comparator for adalimumab as it is the only licensed intervention representing an alternative to systemic therapy; etanercept and ustekinumab are licensed for individuals who are inadequately controlled by, or who are intolerant to, previous systemic therapies. Standard systemic therapies such as methotrexate, ciclosporin and acitretin are not licensed for psoriasis in children and young people. However, it is evident from the UK audit of the assessment and management of psoriasis in children that 19% of children have received systemic drugs (9% methotrexate, 5% acitretin, 4% ciclosporin and 1% dapsone) outside their licensed indications.12,148 The non-biological systemic therapy considered as a comparator in the cost-effectiveness analysis for adalimumab is methotrexate as it is the most widely used systemic therapy in the population of children and young people and was used as a comparator in the M04-717 trial.
If biological treatments are found not to be effective, individuals are usually offered some form of BSC rather than no treatment. Therefore, BSC is considered a relevant comparator for individuals who have exhausted all treatment options including conventional systemic therapy and phototherapy. BSC tends to include a mix of active non-biological systemic therapies such as methotrexate and ciclosporin and palliative care, including phototherapy, even though these treatments may have been proven to be largely ineffective.
The interventions of etanercept, adalimumab and ustekinumab were compared with each other as appropriate to the licensed population. The use of these interventions outside the age constraints of their licence (e.g. the use of etanercept in children aged < 6 years and ustekinumab in children aged < 12 years) was considered relevant in a scenario analysis. The use of other off-label biological treatments such as infliximab outside its licensed indication in adults was not considered. Advice from our clinical expert (Dr Ruth Murphy, personal communication) suggested that it is very unlikely that an unlicensed TNF inhibitor would be used as an alternative to a biological treatment that is licensed and available in this population. Furthermore, there are no RCTs comparing the use of infliximab with the use of any comparator (or placebo) in the population of children and young people. Infliximab also requires intravenous infusion in hospital and our clinical expert suggested that this is not a favourable option in this young population.
The biosimilar of etanercept, namely Benepali (50 mg), is not licensed for use in children and young people. Therefore, the biosimilar was not considered a relevant comparator in the base-case analysis. However, a scenario analysis was considered in which the drug cost of etanercept was reduced by approximately 10% to match the cost of Benepali in adults (£164.00 per prefilled syringe).149
The drug doses for the interventions and comparators considered in the cost-effectiveness analysis are shown in Table 57. These are based on licensed doses for etanercept, adalimumab and ustekinumab and expected doses for methotrexate and BSC. Continuation of treatment was conditioned on response to treatment at the end of the trial period, corresponding to the time point specified in the Summary of Product Characteristics for children and young people. For etanercept and adalimumab, this was 12 and 16 weeks respectively. For ustekinumab, the Summary of Product Characteristics specifies that consideration should be given to discontinuation if there is no response up to 28 weeks. In the analysis, the time point for response to ustekinumab was taken to be 16 weeks, corresponding to its administration at 12 weeks after the dose given at 4 weeks. This is the same time point that was used to assess response to ustekinumab in adults (TA180100). It was assumed that all treatments are used continuously in responders to treatment until treatment withdrawal. Ciclosporin (used as part of BSC) was assumed to have a maximum treatment duration of 2 years.
TABLE 57
Licensed or guideline doses of interventions and comparators used in the economic analysis
Methods
Overview
A de novo decision-analytic model was developed to estimate the cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. The cost-effectiveness model consists of a Markov cohort transition model developed in Microsoft Excel® (2013; Microsoft Corporation, Redmond, WA, USA). The structure of the model is very similar to that used in previous TAs of moderate to severe plaque psoriasis in adults. The model was developed in accordance with the NICE reference case.150 The time horizon of the model extends until individuals reach 18 years of age, when they then become adults and current NICE recommendations for the use of the interventions in adults apply. The length of the time horizon varies by the starting age of individuals in the model. As indicated previously, three starting ages were considered in the model to reflect the restrictions of the marketing authorisation of the interventions.
The outcomes of the model are expressed using QALYs. The QALY provides a summary measure combining estimates of the remaining length of life (life-years) with the associated quality of life. QALYs are derived by multiplying a utility value (quality of life) by the time spent with this utility (length of life). The utility values used in the model were generated from PedsQL trial data using a mapping algorithm to convert them to EQ-5D utility values. The utilities associated with treatment were based on the proportion of individuals in the different PASI response categories (see Health-related quality of life). All costs were considered from the perspective of the NHS and Personal Social Services (PSS). Health-care resource use and cost categories include the cost of treatment (acquisition, administration, monitoring and AE costs) and changes in health service resource use because of loss of response to treatment (see Best supportive care costs).
The parameters for the model were sourced from published literature, information reported in the company submissions and the results of the evidence synthesis described in Chapter 4. Both costs and QALYs were discounted at 3.5% per annum, in line with current NICE guidance.150
Model structure and assumptions
The model consists of four health states: ‘trial period’, ‘continued use’, BSC and death (Figure 10). Individuals enter the model in the trial period and receive one of the three biological interventions or a relevant comparator. The length of the trial period is dependent on the intervention and can last from 12 weeks for etanercept to 16 weeks for adalimumab and ustekinumab, corresponding to the time point at which response to treatment is assessed. The cycle length in the model corresponds to 28 days (4 weeks), which takes account of the different lengths of time spent in the trial period.
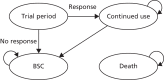
FIGURE 10
Schematic of the model structure.
At the end of the trial period, individuals are assessed as responders or non-responders to treatment based on PASI response rates. PASI response in the base-case analysis is taken to be PASI 75, that is, response is assessed based on whether or not an individual achieves a 75% reduction in baseline PASI score. Individuals who do not have an adequate response to treatment at the end of the trial period move to BSC. Individuals who are considered responders to treatment transition to the health state of continued use, remaining in this state until they withdraw from treatment and move to BSC. During the period of continued use, individuals continue to receive the active therapy and are assumed to maintain their level of PASI response until treatment discontinuation from any cause, such as lack of efficacy, the presence of AEs or non-compliance to treatment (modelled together as an overall risk of all-cause withdrawal).
On treatment discontinuation (in either the trial period or the continued use state), individuals transition to BSC. BSC consists of non-biological supportive therapies. The only transition out of the BSC state is to the ‘death’ state. Death is an all-cause mortality state to which transition is possible from any health state. Mortality is not conditioned on treatment or treatment response. Mortality rates by age were sourced from life tables in England and Wales for the years 2013–15151 and averaged across sexes.
Effectiveness data
The measure of treatment effectiveness used in the model was the proportion of individuals achieving a specific threshold of PASI response relative to baseline. Relative change in PASI response is the most widely reported outcome in clinical trials and has been used as the main outcome in previous models in adults. The PASI response rates used in the model were taken directly from the children and young people efficacy estimates from the NMA that incorporated all relevant adult evidence (see Chapter 4, Framework of analysis for informing the relative efficacy of the interventions). Scenario analyses were also conducted in which the results from the unadjusted baseline constrained model with minimum adult evidence (see Chapter 4, Framework of analysis for informing the relative efficacy of the interventions) were applied in the model; partial comparisons with direct trial data and the indirect comparison (see Chapter 4, Indirect treatment comparison) were also incorporated in scenario analyses for completeness. None of the three trials of biological therapies in children and young people with psoriasis required previous failure on non-biological systemic therapy as an inclusion criterion. Therefore, it was assumed in the model that treatment effectiveness is independent of failure on non-biological systemic therapy prior to starting biological therapy. It is unknown how the position on the care pathway is likely to affect treatment effectiveness. In the base-case analysis, PASI 75 response rates were taken as the measure of effectiveness for treatment continuation. Individuals who meet the threshold of PASI 75 are classified as responders at the end of the trial period and are assumed to maintain their response for as long as they are in the health state of continued use. In a separate scenario analysis, the threshold of PASI 50 was taken as the measure of effectiveness for treatment continuation.
The PASI response rates from the NMA were also used in the model to inform the HRQoL utility values. Gains in utility associated with treatment were conditioned on PASI response rates (see Chapter 5, Health-related quality of life in the York model and subsequent appraisals), an approach that has been taken in previous models for the treatment of psoriasis in adults. PASI response rates for BSC were assumed to be equivalent to those for placebo in the NMA.
In the absence of data to model time-varying transition probabilities, response rates were assumed to be constant per cycle in the model. The response rates used to inform the model are presented later in this chapter (see Table 70). The uncertainty in the predicted response rates from the NMA was reflected in the model by directly exporting the simulated posterior distributions from the Markov chain Monte Carlo analysis in WinBUGS to the cost-effectiveness analysis, preserving any correlations in the data.
TABLE 70
Summary of parameters used in the model
Treatment withdrawal rates
Responders to treatment were assumed to maintain their response until treatment discontinuation. Discontinuation was modelled as an overall risk of withdrawal from any cause, such as lack of efficacy, the presence of AEs or non-compliance to treatment. Previous TAs in adults assumed a constant withdrawal rate of 20% per annum for all treatments.
A literature search, described in Chapter 3, was conducted with the aim of identifying registry data on long-term treatment response to biologics in children and young people with psoriasis. Two registries were identified: Child-CAPTURE90 (Netherlands) and DERMBIO92 (Denmark). However, none of the published studies from these registries allowed the estimation of long-term withdrawal rates in individuals who are responders to treatment; in addition, the DERMBIO registry included only a small number of children. The data indicated that there was no significant predictive relationship between age and treatment continuation, which may suggest that treatment withdrawal rates used in the adult population can be extrapolated to children and young people in the absence of any alternative source of data. Data from the DERMBIO registry suggest that the withdrawal rate on biological therapies is constant over the treatment period (with no obvious plateau),92 which supports the use of a constant withdrawal rate over time.
A recent study on the long-term drug survival rates of four biologics (adalimumab, etanercept, infliximab and ustekinumab) based on data from the UK BADBIR audit of 3523 biologic-naive adult patients indicated that loss of efficacy is a major reason for treatment discontinuation, with efficacy decreasing from 77% in the first year of use to 53% in the third year of use.94 This is consistent with a withdrawal rate of 20% per annum, which has been used in previous TAs in adults. This study also suggested that there may be differences in the withdrawal rate by treatment, with ustekinumab having a significantly higher survival rate than adalimumab and etanercept. However, the study did not distinguish between discontinuation because of a lack of treatment response in the short term, that is, during the initial trial period, and discontinuation because of a lack of treatment response in the long-term for patients who are responders to treatment. Therefore, the differences in withdrawal rates by treatment may reflect the higher efficacy of ustekinumab than adalimumab and etanercept, rather than reflecting differences between the treatments conditional on response at the initial assessment point.
In the absence of sufficient evidence on the long-term withdrawal rates in children and young people, and given that observational data generally suggest that a constant 20% annual withdrawal rate is a reasonable assumption in adults, the same withdrawal rate was assumed in the model (this rate equates to a 28-day discontinuation rate of 1.70% per cycle).
All-cause mortality
All-cause mortality was incorporated in the model by applying a risk of death during each cycle. The mortality risk was assumed to be independent of response status or treatment received. A common mortality risk was thus assumed for all patients based on the general population mortality risk. The general population mortality risk was obtained from sex-specific life tables for England and Wales for the period between 2013 and 2015, with the risk averaged across males and females, assuming equal proportions.151
Health-related quality of life
Review of utility data in children and young people with psoriasis
A systematic literature review was conducted to identify utility values for plaque psoriasis in children and young people. The aim of the search was to identify any studies that reported utility values or other measures of HRQoL that could be converted into utility values specifically for the population of children and young people.
The search strategy was developed in MEDLINE (via Ovid) by an information specialist with input from the project team. The strategy included terms for psoriasis combined, using the Boolean operator AND, with terms for quality of life/utilities or named instruments. No language, geographical or date limits were applied. A search filter to limit retrieval to quality-of-life studies was used when available. The search strategy was adapted for use in the other resources searched. Full search strategies can be found in Appendix 1.
The following databases were searched on 12 July 2016: MEDLINE [including MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)], the Cost-effectiveness Analysis (CEA) Registry [see https://research.tufts-nemc.org/cear4/ (accessed 10 August 2017)] EMBASE, PubMed and the School of Health and Related Research Health Utilities Database (ScHARRHUD) [see www.scharrhud.org/ (accessed 10 August 2017)].
The Health Economics Research Centre (HERC) database of mapping studies from the University of Oxford152 was also searched to identify any suitable mapping algorithms that would allow conversion of clinical measures routinely collected in studies of psoriasis in children and young people into utility values.
The results from the searches were imported into an EndNote X7 library and deduplicated. After deduplication, 286 records in total were identified. The titles and abstracts were assessed independently by two reviewers for inclusion and any discrepancies were resolved by consensus. None of the titles identified reported utility values collected in children and young people with psoriasis. The search of the HERC mapping algorithm database identified one study on the development of a mapping algorithm to estimate EQ-5D-Y (EuroQol-5 Dimensions for Youth) utility values from PedsQL general core scales.153 The PedsQL is a generic instrument for measuring HRQoL in children and adolescents with acute and chronic health conditions. The PedsQL measures core dimensions of health as delineated by the WHO, as well as role (school) functioning. The four multidimensional scales are physical functioning (eight items), emotional functioning (five items), social functioning (five items) and school functioning (five items).19 The EQ-5D-Y is the youth version of the EQ-5D, which has been specifically adapted in terms of language for children aged 8–11 years and adolescents aged 12–18 years. In the absence of a tariff set specifically for the EQ-5D-Y, the authors153 applied the UK national tariff for the valuation of the EQ-5D-3L (EuroQol-5 Dimensions three-level version) to generate utility values from the EQ-5D-Y instrument.154
Khan et al.153 assessed different mapping methods for estimating EQ-5D-Y health utilities from PedsQL response scores. The study used data collected in a cross-sectional survey conducted in four secondary schools in England among children aged 11–15 years. The sample on which the mapping models were estimated included 559 children and the validation sample included 337 children. Children in the full study sample (n = 896) were on average aged 13.3 years (standard deviation 1.3 years), 54% were male and approximately 40% were of non-white ethnicity. The authors explored both direct and response mapping approaches to predict EQ-5D-Y utility values, as well as a number of functional forms, including ordinary least squares (OLS) regression, generalised linear models, two-part logit–OLS regression, censored least absolute deviation and Tobit regression. Model performance was assessed on the validation sample and models were re-estimated on the full study sample. Table 58 presents the two best-fitting models for the mapping algorithm. These correspond to the models estimated using OLS regression with (1) age and sex terms included as regressors and (2) excluding age and sex terms as regressors.
TABLE 58
Best-fitting mapping algorithms from the PedsQL to EQ-5D-Y based on the study by Khan et al.
The two models were considered to have similar prediction accuracy for mean EQ-5D-Y values. Model 1, which included age and sex as regressors, had a better fit across a wider range of EQ-5D-Y values than model 2. Model 2 reported a better fit for the EQ-5D-Y utility score range of 0.8–1.0 category. There are a number of potential limitations to the use of this algorithm to predict EQ-5D-Y utilities. The sample on which the models were estimated consisted of healthy children aged 11–15 years, which may limit the predictive accuracy in sicker populations or populations outside this age range. The authors recognise that there is a need for further validation and testing of the algorithm but, in the absence of an alternative source, it remains a useful tool for estimating EQ-5D-Y utility values in situations in which only the PedsQL has been administered.
Utility data reported in company submissions
Health-related quality-of-life assessments were carried out in the etanercept trial (2003021149), the ustekinumab trial (CADMUS72) and the adalimumab trial (M04-71742) using the CDLQI and the PedsQL at selected time points. EQ-5D or EQ-5D-Y values were not collected in any of the trials. Therefore, the only way to include EQ-5D utility values in the model was by mapping from either CDLQI scores or PedsQL scores. The literature review described earlier did not identify any studies that estimated the relationship between CDLQI scores and EQ-5D values, whereas the study by Khan et al.153 was the only study that estimated the relationship between PedsQL scores and EQ-5D values. The AG requested from the companies access to individual patient data (IPD) for PedsQL domain scores at baseline and follow-up by category of PASI response and PedsQL summary scores at the domain level by response category. The AG did not receive access to IPD; however, Janssen (ustekinumab) submitted aggregated summary data (mean and standard deviation) from the CADMUS trial for the PedsQL subscale and total scale scores by treatment arm (placebo and ustekinumab standard dose) and PASI response category at 12 weeks (< 50, 50–74, 75–89, ≥ 90) for baseline and 12, 28 and 52 weeks.
Utility estimates used in the model
The utility values associated with treatment in previous models in adults were based on the proportion of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) and the change in utility from baseline associated with the PASI response categories. Therefore, PASI response rates from the NMA were assumed to be a perfect proxy for change in utility arising from treatment.
The relationship between utility and PASI response was estimated in previous TAs in adults using either DLQI data mapped onto EQ-5D utility values or directly from EQ-5D data collected in the trials. In the population of children and young people, the only possibility of obtaining EQ-5D values was by mapping from PedsQL scores to EQ-5D-Y values, as described earlier. Without access to the IPD, which would allow full uncertainty to be reflected in the values, the mapping algorithm was applied to the summary scores at the domain level from the CADMUS trial.
Validation of the algorithm was performed by examining data reported in a study by Varni et al.,155 which compared self-reported HRQoL (based on PedsQL scores) between paediatric patients with moderate to severe plaque psoriasis and a healthy population sample. The sample used to represent the psoriasis population corresponded to individuals in the main efficacy trial for etanercept (n = 208, age 4–17 years) and measurements of PedsQL scores at baseline were pooled across the two treatment arms (etanercept and placebo). The healthy population sample was taken from a US children’s health insurance programme evaluation (n = 5079) open to children and young people aged 2–16 years. Table 59 summarises the PedsQL subscale scores reported in Varni et al.155 for the psoriasis and healthy populations, alongside the estimates obtained by applying the two best-fitting mapping algorithms to obtain EQ-5D-Y utility values (model 1 includes age and sex terms whereas model 2 excludes these variables).
TABLE 59
Application of the mapping algorithm to estimate EQ-5D-Y utilities in paediatric populations
The EQ-5D utility estimates were higher in the healthy population than in the population with psoriasis, irrespective of the model used to map PedsQL scores to EQ-5D-Y values. The distinction between the models was minimal, especially in the psoriasis population: model 1 provided slightly higher utility values than model 2 (0.6% and 2.5% higher in the psoriasis and healthy populations respectively). Model 2 was subsequently used in the base-case analysis as the reference category for the variable sex was unclear in the study by Khan et al.153
The mapping algorithm (model 2 in Table 58) was used to estimate change in EQ-5D-Y utility values from baseline based on PedsQL data from the ustekinumab trial (CADMUS) at baseline and 12 weeks’ follow-up (the time point at which response to treatment was assessed in the trial and blinding of randomised subjects in the trial was terminated; after this point crossover between treatment arms was possible). The mean change in EQ-5D-Y values between baseline and week 12 was estimated for individuals with different levels of PASI response. Table 60 reports the EQ-5D-Y utility values estimated for the base-case-analysis, with the placebo and treatment arms pooled.
TABLE 60
Baseline utility and mean change in utility by PASI response, estimated from CADMUS trial PedsQL data mapped onto the EQ-5D-Y
The baseline utility estimate is similar to that derived from the etanercept trial (20030211) (0.864 in Table 59) and is lower than the general healthy population estimate of 0.913 based on the study by Varni et al.155 The mean changes in EQ-5D utility from baseline by PASI response category are much smaller than the corresponding changes in EQ-5D utility observed in previous TAs in adults. For example, the EQ-5D changes in utility by PASI response category in the York model of adults were 0.05 for PASI < 50, 0.17 for PASI 50–74, 0.19 for PASI 75–89 and 0.21 for PASI ≥ 90.
To examine whether or not the changes in EQ-5D-Y utility values were accompanied by similar changes in other measures of HRQoL in the population of children and young people, the changes in EQ-5D-Y values were compared with reported CDLQI values by PASI response (Table 61). A comparison of EQ-5D and DLQI values by PASI response in adults is also shown in Table 61 (taken from TA180100 for ustekinumab, which was the only TA in adults that reported both outcomes). The mean changes in CDLQI score by PASI response in the CADMUS trial are much smaller than the mean changes in DLQI score in TA180,100 which is consistent with the smaller mean changes estimated for the EQ-5D-Y in the paediatric population than for the EQ-5D in adults. These differences, however, should be interpreted with caution as CDLQI and DLQI scores are not directly comparable and the number of observations was much smaller in the population of children and young people than in the adult population in TA180.100
TABLE 61
Mean change from baseline in CDLQI/DLQI scores and EQ-5D/EQ-5D-Y utilities by PASI response
The EQ-5D-Y/EQ-5D utility estimates suggest that improvements in HRQoL associated with reductions in PASI response rates are of a much smaller magnitude in children and young people than in adults; however, the evidence is highly uncertain because of the small sample size and the limited data available to validate the findings. In the absence of an alternative source to estimate EQ-5D values for the model, these values were used in the base-case analysis. It is important to highlight a number of limitations of this approach. First, the use of a mapping algorithm to estimate utilities introduces uncertainty compared with direct EQ-5D measurement. Second, the Khan et al.153 mapping algorithm has not been validated in children aged < 11 years or in a population with psoriasis. Third, the CADMUS trial, from which the PedsQL data that were mapped to EQ-5D utilities were sourced, excluded children aged < 12 years; therefore, it remains uncertain whether or not the mapped utilities are reflective of this population. Fourth, in populations aged < 12 years, there may be issues with lack of agreement or consistency between self-reported and proxy (parent)-reported measurements.156 Therefore, even if PedsQL data were available for younger children, the mapping algorithm might not consistently perform for self-reported and parent-reported measurements of the instrument. Finally, Khan et al.153 used the EQ-5D-3L value set as a proxy for EQ-5D-Y in the absence of an alternative tariff set, but this approach is currently not recommended.157 These limitations reduce the robustness of the utility estimates used in the model.
There might be other potential benefits of treatment that fall outside the QALY estimation. First, children and young people may miss schooldays to attend health-care appointments and may be absent for longer periods from school while experiencing symptoms. This can have a negative impact on their education/academic achievements and, in future, their ability to gain employment. It may also affect their social and psychological health through the reduced ability to participate in social and leisure activities and sport. Second, early treatment of children and young people with biological agents may prevent long-term multisystem morbidity (e.g. hypertension, cardiovascular disease, depression), which has a higher prevalence in adults with psoriasis than in the general population.158 Finally, there may also be other aspects of HRQoL that are outside the perspective defined by NICE’s reference case,150 namely the potential impact on the HRQoL of carers of children and young people with psoriasis if treatment with biologics reduces the spillover disutility of illness by improving patients’ outcomes. The impact on carers may also extend to a reduced ability to participate in normal activities, both work- and non-work related. Because of an absence of quantitative estimates of the impact on the HRQoL of children and young people with psoriasis receiving any of the interventions and the potential benefits to their carers, it was not possible to incorporate them into the economic analysis. Any attempt to add arbitrary values to the utility estimates, which are already highly uncertain, would introduce further uncertainty.
Given the uncertainty surrounding the utility estimates for children and young people, scenario analyses were conducted using utility estimates from previous TAs in adults for etanercept, adalimumab and ustekinumab. Table 62 summarises the utility estimates considered in the scenario analyses.
TABLE 62
Baseline utility and mean changes in utility by PASI response used in the base-case and scenario analyses
Utility estimates by health state
The HRQoL utility values were applied in the model based on PASI response to treatment. The utility values in the trial period and period of continued use for each treatment were based on the proportions of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) and the change in utility from baseline associated with a PASI response. During the trial period, individuals are assigned utility values based on treatment response at the end of the trial period:
where u00 is the utility gain for individuals not achieving a PASI 50 response; u50 is the utility gain for individuals achieving a PASI 50 response but not a PASI 75 response; u75 is the utility gain for individuals achieving a PASI 75 response but not a PASI 90 response; u90 is the utility gain for individuals achieving a ≥ PASI 90 response; and is the probability of a PASI XX response with treatment.
During the period of continued use, individuals are assigned utility values based on maintaining a treatment response at the end of the trial period, which is based on meeting the minimum of a PASI 75 response:
Individuals who discontinue treatment progress to BSC. The utility associated with BSC was based on the proportion of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) for BSC (assumed to be equal to the placebo response from the NMA):
A scenario analysis was considered in which the utility of individuals receiving BSC was set to be equal to baseline utility, that is, there are no health benefits from BSC.
On entering the death state, individuals are assigned a utility value of zero. Table 63 summarises the utility estimates applied in the base-case analysis by treatment and health state.
TABLE 63
Utility values by treatment and health state used in the base-case analysis
Given the paucity of evidence on AEs in children and young people receiving biological treatment for psoriasis, and similarly to the majority of previous TAs in adults, no disutility from treatment was applied in the model.
Resource utilisation and costs
Resource use and costs included in the model correspond to direct NHS costs and include treatment acquisition costs, administration costs, monitoring costs, costs associated with AEs and the costs of BSC. Costs were sourced from NHS reference costs 2014–15,159 the Monthly Index of Medical Specialities (MIMS),160 the BNF,161 Curtis and Burns and published literature. When costs were not available for 2015–16, they were inflated to 2014–15 prices based on the Hospital & Community Health Services Index published in Curtis and Burns. The systematic literature review described in Chapter 3 (see Methods for the synthesis of evidence of clinical effectiveness) considered broad search terms to capture resource utilisation and costs associated with the treatment of psoriasis in the population of children and young people. The search identified five studies162–166 that estimated resource use and the costs of biological therapies in psoriasis from insurance claim databases, but on further examination of the populations included in the studies it became clear that only adults were considered in the databases. In addition, the studies used data from US insurance databases, which are unlikely to reflect health-care resource use in the UK.
Given the lack of data on resource use and the costs of treatment for psoriasis in children and young people, previous NICE TAs for adults were hand-searched to identify relevant resource use categories and potential sources of resource use estimates and unit costs. These were tabulated and sent to our clinical advisor (see Chapter 5, Resource use and costs in the York model and subsequent appraisals), who then worked with us to help establish the transferability of the adult data and resource use assumptions to the population of children and young people.
According to our clinical advisor, the management of psoriasis in children and young people is very similar to that in adults. Therefore, it seems reasonable to assume that the resource use associated with the administration of the treatments and monitoring costs in children and young people would be similar to those used in previous TAs in adults. The assumptions used for resource use and costs for each of the cost categories are described in the following sections.
Drug acquisition costs
Table 64 details the dose and frequency of administration for each treatment and comparator, including ciclosporin, which forms part of BSC, and the unit costs associated with each treatment.
TABLE 64
Drug acquisition costs in children and young people
The dosages of the biological therapies were taken from the Summaries of Product Characteristics.167–169 For methotrexate and ciclosporin, which are currently not licensed for paediatric use, the dosages were sourced from published literature78,170,171 and confirmed with our clinical advisor to ensure that they reflected UK clinical practice in this population. Methotrexate can be administered orally or injected subcutaneously or intramuscularly. In the model it was assumed that 72% of individuals are given methotrexate in oral solution and 28% in injectable solution, which reflects the distribution of administration identified in the UK psoriasis audit of the use of systemic treatments in children and young people.148 Therefore, the unit cost per mg for methotrexate is a weighted average of the unit cost per mg of the oral and injectable solutions (i.e. £0.71/mg). Unit costs were sourced from MIMS160 and supplemented with data from the BNF.161
Figure 11 illustrates the number of doses administered in the first five cycles of the model for each treatment based on the licensed dose. Adalimumab is administered at weeks 0 (baseline) and 1 and then every 2 weeks thereafter until response assessment at the end of week 16. If individuals are responders to treatment they continue to receive adalimumab every 2 weeks until treatment withdrawal (highlighted in grey). Ustekinumab is administered at weeks 0 and 4 and then every 12 weeks thereafter, with response assessment at week 16. Etanercept and methotrexate are administered weekly, with response assessment at weeks 12 and 16 respectively.
The dosages of the biological treatments are dependent on patient weight. The median weight by age and sex in the population of children and young people was extracted from the Royal College of Paediatrics and Child Health’s school-age growth charts.172 Table 65 shows the weights used in the model by age. These were based on an average of the weight of boys and girls (and when the weight estimate in the growth chart did not correspond to an integer, the next-highest integer was used).
TABLE 65
Median weight by age used in the model
The weight by age was used to estimate the correct dosage of each treatment and the corresponding cost. Table 66 summarises the dosages used in the model for each treatment by age and the corresponding costs per dose. Following clinical advice it was assumed that the vial with the lowest dose available would be used to allow administration of a single dose in the paediatric population. This inevitably results in wastage of the remainder of the vial. For example, for individuals who weigh < 60 kg the full cost of the 45-mg vial of ustekinumab is assumed as the remaining product in the vial cannot be stored. Vial splitting across individuals was considered unlikely because in most cases the majority of the vial is used for a single patient and treating patients together is less likely to occur in this population because of low patient numbers. Therefore, the cost per dose was fixed for adalimumab and ustekinumab (£352.14 and £2147.00 respectively). For etanercept, the 25-mg vial (£89.38) is used for children aged < 10 years whereas the 50-mg vial (£178.75) is used for those aged ≥ 10 years.
TABLE 66
Drug dosages and cost per dose by age in the model
Drug administration costs
Adalimumab, etanercept and ustekinumab were assumed to be self-administered. In the case of younger children it was assumed that a parent or carer would administer the subcutaneous injection. Subcutaneous injections were assumed to incur administration costs only for nurse training for self-administration (or parent/carer administration) in the induction phase. In line with previous TAs in adults, this was assumed to require 3 hours of nurse time, which was costed based on the cost per working hour of a band 5 hospital nurse with qualifications (£43 per hour). A cost of £129 was applied in the first cycle of the model for the administration of the biologics.
Monitoring costs
Table 67 summarises the resource use assumptions made in relation to monitoring and the corresponding unit costs applied in the model. In the absence of evidence specifically relating to the population of children and young people, resource use estimates associated with monitoring and routine laboratory tests for biological and non-biological systemic treatments were taken from NICE CG153,173 which used similar assumptions to those in the original York model (TA10397) and subsequent NICE appraisals of biological treatments.98–102
TABLE 67
Monitoring resource use and unit costs
Individuals on biological therapy were assumed to undertake a series of tests during the initial trial period, namely a full blood count, liver function test and urea and electrolytes test. During the trial period the tests were assumed to be carried out during two routine outpatient visits that occur at treatment initiation and at the end of the trial period (treatment response assessment visit). As methotrexate was not included as a comparator in CG153173 (only as part of BSC), it was assumed that the resource use for the monitoring of methotrexate in the trial period is the same as that for biological treatment. In the maintenance period (corresponding to the health state of ‘continued use’), individuals on systemic therapies were assumed to be monitored once every 3 months.173 The unit costs for glomerular filtration rate and outpatient visits were taken from NHS reference costs 2014–15,159 whereas the costs of the remaining monitoring items were inflated to 2014–15 prices based on estimates presented in TA103.97
The costs of tests undertaken solely to screen individuals for eligibility for treatment were excluded from the analysis, namely chest radiography, tests for tuberculosis or biopsies of lesions atypical of psoriasis. These costs were also excluded in previous appraisals in adults. The cost of folic acid used in conjunction with methotrexate to prevent side effects was also excluded from previous appraisals as the annual cost of this drug is very low (< £1). Our clinical advisor indicated that children and young people would be tested for herpes zoster before treatment initiation; however, as this test would be performed on every patient not immune to the virus regardless of treatment, it was excluded from the analysis. The costs of liver biopsy and type III procollagen peptide (PIIINP) monitoring for the purpose of assessing liver function in individuals treated with methotrexate were also excluded from the analysis based on clinical advice; liver biopsy is seldom conducted in children and young people given its invasiveness, whereas PIIINP is a marker of growth in this population rather than of hepatic toxicity.
Best supportive care costs
Best supportive care corresponds to the management of individuals after failure of conventional systemic therapies. BSC is also considered a relevant comparator to biological treatments. If biological treatments are found not to be effective, individuals are usually offered some form of BSC rather than no treatment. BSC tends to include a mix of active non-biological systemic therapies such as methotrexate and ciclosporin and palliative care, including phototherapy, as well as outpatient visits and hospitalisations to manage disease flare-ups.
The resource use and costs associated with BSC have represented a significant area of uncertainty in the analysis of the cost-effectiveness of biological treatments for moderate to severe psoriasis in adults. In TAs prior to CG153,97–100 the definition of BSC in terms of resource use and costs was restricted to outpatient visits and hospitalisations to manage the symptoms of psoriasis, with these largely informed by assumptions and clinical opinion. In CG153,173 the definition of BSC was expanded to also include non-biological systemic treatments, phototherapy and attendance at tertiary day centres. As discussed previously (see Chapter 5, Resource use and costs in the York model and subsequent appraisals), this guideline used estimates of resource use from observational studies in the UK143 and the Netherlands144 but also relied heavily on clinical opinion and assumptions. In the absence of evidence for children and young people, the definition of BSC from CG153173 was used in the model, with input from our clinical advisor on the appropriateness of the assumptions for a younger population.
Table 68 summarises the resource use assumptions for BSC by category of cost in CG153173 and those applied in the model, alongside the associated unit costs. Unit costs were sourced from the BNF,161 MIMS160 and NHS reference costs 2014–15.159 The relative proportions of individuals on active treatment with methotrexate and ciclosporin were modified from those used in CG153173 based on clinical opinion that children and young people are less likely to be managed with ciclosporin than adults because of the renal toxicity of the drug. Data from a UK psoriasis audit on the use of systemic treatments in children and young people were used to inform the relative proportion of individuals on methotrexate and ciclosporin.148 In CG153, it was assumed that 90% of individuals receiving BSC would be on active treatment with systemic drugs. However, in the audit, 53 patients were treated with non-biological systemic treatments, of whom 25 patients were treated with methotrexate and 12 with ciclosporin. Therefore, instead of assuming that individuals are equally distributed between methotrexate and ciclosporin, a ratio (25/37 and 12/37 for those on methotrexate and ciclosporin respectively) for each treatment was applied to the overall proportion of 90% to reflect the distribution of children and young people receiving these treatments in the audit. The corresponding proportions of individuals assumed to receive methotrexate and ciclosporin as part of BSC were 61% and 29%, respectively. As in CG153,173 treatment with ciclosporin was assumed to be discontinued after a maximum duration of 2 years (because of the increased risk of renal toxicity). Monitoring costs associated with the use of these non-biological systemic therapies were applied in the model as presented in Monitoring costs.
TABLE 68
Resource use assumptions and unit costs for BSC in CG153 and the current analysis
In line with CG153,173 16% of the population were assumed to undergo 24 sessions of phototherapy per year (NBUVB) and five outpatient visits per annum were assumed for the 10% of individuals not managed with systemic therapies. All individuals were assumed to incur the costs of five visits per annum to a specialist dermatology day centre, in line with CG153.173
The resource use associated with hospitalisations for individuals on BSC was identified as an area of high uncertainty and a key driver of cost-effectiveness in the previous TAs in adults. The number of bed-days assumed in CG153173 (26.6 days per year) was based on the average LOS for psoriasis patients with a baseline PASI of 10–20 points taken from a UK observational study145 combined with the average number of hospitalisations for individuals at high need (one hospitalisation per year) and very high need (2.55 hospitalisations per year) from a Dutch observational study.144 The total of 26.6 days of hospitalisation per annum was considered by the NICE Appraisal Committees for TA350101 (secukinumab) and TA368102 (apremilast) in adults to be too high. Our clinical advisor suggested that hospitalisations in children and young people are very rare. This is largely because children and young people have not yet developed the comorbidities that often lead to hospitalisations in adults with psoriasis. Therefore, in the base-case analysis it was assumed that children and young people do not incur any inpatient stays. In separate scenario analyses, estimates of 26.6 days of hospitalisation per annum173 and 6.49 days of hospitalisation per annum144 were considered.
Adverse event costs
As discussed in Chapter 5 (see Resource use and costs in the York model and subsequent appraisals), only one previous TA in adults101 considered the costs of hospitalisations resulting from AEs in the cost-effectiveness analysis. The AEs that were assumed to lead to relevant resource use consumption (i.e. those leading to hospitalisations) in this evaluation were (1) NMSC, (2) malignancies other than NMSC and (3) severe infections. The rates of AEs as reported in the literature (for adalimumab, etanercept, ustekinumab and infliximab) and from trial data (for secukinumab) were applied to each treatment arm as per the rates of these events occurring.
The safety data from the clinical trials of biological drugs for the treatment of severe-to-moderate psoriasis (see Chapter 3, Safety of adalimumab, Safety of etanercept and Safety of ustekinumab) suggested that there was little difference in the short- and long-term rates of AEs between trial arms, with the potential exception of etanercept, for which a higher rate of infections (not statistically significant) was observed than for placebo. However, the trial data included a small number of observations for each treatment and a limited follow-up period (from 52 weeks for adalimumab46,47 to 312 weeks for etanercept).49,52,80 Observational studies in children and young people with psoriasis76,77 (see Chapter 3, Additional observational evidence) did not report any increase in infections or SAEs associated with the use of biological therapies.
Given the paucity of robust evidence on the incidence of AEs in children and young people with moderate-to-severe psoriasis, the costs of these were not included in the base-case analysis. However, scenario analyses were conducted to explore the impact on the cost-effectiveness results of including the costs associated with hospitalisations resulting from serious infections and malignancies (both NMSC and other). The rates of AEs were sourced from TA350101 and supplemented with data from Dixon et al.174 for methotrexate, whereas the unit costs were taken from NHS reference costs 2014–15.159 Table 69 summarises the adverse event rates applied in the model, alongside the corresponding unit costs.
TABLE 69
Adverse event rates applied in the model
The costs of AEs associated with biological therapies and methotrexate were applied in the model to individuals while on treatment. Individuals treated with BSC were assumed not to develop AEs.
Analytical methods
Base-case analysis
The expected costs and QALYs of the interventions and comparators were determined for each population and the relative cost-effectiveness was established using standard decision rules and reported using ICERs as appropriate. The ICER examines the additional cost that one treatment option incurs over another and compares this with the additional health benefits to give the additional cost of the treatment for each additional QALY gained. When more than two treatment options are being compared, the ICERs are calculated using the following process:
- The treatment options are ranked in terms of mean QALYs (from the least effective to the most effective).
- If a treatment option is more costly and less effective than any other option, then this treatment is said to be dominated and is excluded from the calculation of the ICERs.
- The ICERs are calculated for each successive alternative, from the least effective to the most effective. If the ICER for a given treatment option is higher than that of any more effective option, then this treatment option is ruled out on the basis of extended dominance.
- Finally, the ICERs are recalculated, excluding any treatment options that are ruled out by principles of dominance or extended dominance.
The resulting ICERs provide the basis for establishing which treatment appears optimal based on cost-effectiveness considerations. Guidance from NICE150 suggests that an incremental cost per additional QALY of around £20,000–30,000 is considered to represent an appropriate threshold for the health opportunity costs to the NHS.
The ICER comparing all interventions and comparators relates to a situation in which the decision-maker can choose only one of the treatment options. However, in psoriasis, as indicated previously, if an individual patient does not respond to or tolerate one of the biological therapies, an alternative one is usually tried. This means that treatments are usually trialled on an individual basis until an effective option is found. The ICERs comparing each intervention with BSC (after systemic therapy) or methotrexate (before systemic therapy) are also presented, to indicate the optimum ordering of treatments in terms of their cost-effectiveness. The most cost-effective order in which to give the therapies based on total expected costs and QALYs associated with each treatment option is dependent on the cost-effectiveness threshold.
Probabilistic sensitivity analysis was used to represent uncertainty in the cost-effectiveness results. The effectiveness data were entered as simulated posterior distributions from the Markov chain Monte Carlo analysis to reflect uncertainty in the mean estimates. Monte Carlo simulation was used to propagate the uncertainty in the input parameters over 10,000 draws, from which mean costs and QALYs were then obtained by averaging over the 10,000 simulations. The probability that a treatment is first in the sequence was also estimated.
Differences in the marketing authorisations of the interventions by age and the positioning of adalimumab before non-biological systemic therapy means that the comparative cost-effectiveness of the interventions needs to be evaluated by age and before or after use of systemics. The relevant comparator also depends on the position of the particular intervention in the pathway. Before systemic therapy, methotrexate is the relevant comparator (as the current standard of care), whereas after systemic therapy BSC represents the most relevant comparator. Three base-case populations are presented:
- children and young people aged 4–17 years with adalimumab compared with methotrexate, that is, as a second-line therapy in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies
- children and young people aged 6–11 years with adalimumab and etanercept compared with BSC and with each other, that is, as third-line therapy in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies
- children and young people aged 12–17 years with adalimumab, etanercept and ustekinumab compared with BSC and with each other, that is, as third-line therapy in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
Table 70 summarises the input parameters used in the base-case analysis.
Scenario analysis
A number of alternative scenarios were considered in which the assumptions used as part of the base-case analysis were varied. These analyses were undertaken to assess the robustness of the base-case results to variation in the assumptions and sources of the data used to populate the model. Table 71 summarises the alternative scenarios considered. For each element, the position in the base-case analysis is outlined, alongside the alternative assumptions applied. The cost-effectiveness of the interventions was considered under each of the scenarios for each of the licensed populations.
TABLE 71
Details of the key elements of the base-case analysis and the variations used in scenario analyses
Results
Results of the base-case cost-effectiveness analysis
Table 72 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy. Adalimumab is more costly (additional cost of £27,084) but also more effective than methotrexate (incremental gain in QALYs of 0.088). The resulting ICER is £308,329 per QALY gained. The small incremental gain in QALYs for adalimumab compared with methotrexate is a result of the modest utility increments in EQ-5D-Y for the different PASI response categories (< 50, 50–74, 75–89, ≥ 90). The average proportion of individuals achieving a PASI 75 response is 79% for adalimumab compared with 49% for methotrexate but the utility gains for individuals achieving a PASI 75–89 response and PASI ≥ 90 response are very small at 0.0340 and 0.0810 respectively. Therefore, the difference in effectiveness translates into a small utility gain while on treatment with adalimumab compared with methotrexate. The difference in total costs for adalimumab compared with methotrexate is driven by the difference in treatment costs: adalimumab has a cost of £704.28 per 4-week cycle in the model (i.e. £352.14 per dose every 2 weeks) whereas methotrexate has a cost of approximately £60 per 4-week cycle. The difference in treatment costs is partly offset by the greater efficacy associated with adalimumab, which results in lower costs associated with BSC (i.e. less time spent in BSC) for non-responders compared with higher costs on BSC with methotrexate, but this offset is not sufficient to outweigh the difference in treatment costs. The probability that adalimumab is cost-effective at a threshold of £30,000 per additional QALY is zero.
TABLE 72
Base-case probabilistic results for adalimumab as an alternative to systemic therapy
Table 73 presents the cost-effectiveness results for the interventions after failed systemic therapy by age group. The difference by age group reflects the fact that ustekinumab does not have marketing authorisation for use in children and young people aged <12 years. For the younger age group of 6–11 years, adalimumab is the most effective treatment (8.890 QALYs), followed by etanercept (8.813 QALYs) and BSC (8.710 QALYs). In terms of costs, adalimumab is the most costly treatment (£57,251), followed by etanercept (£43,808) and BSC (£36,406). Based on a fully incremental analysis, the ICER of etanercept compared with BSC is £71,903 per additional QALY, whereas the ICER of adalimumab compared with etanercept is £174,519 per additional QALY. The individual pairwise ICERs for etanercept and adalimumab compared with BSC are £71,903 and £115,825 per additional QALY respectively.
TABLE 73
Base-case probabilistic results for interventions after failed systemic therapy
For children and young people aged 12–17 years, ustekinumab is the most effective treatment (4.960 QALYs), followed by adalimumab (4.950 QALYs), etanercept (4.887 QALYs) and BSC (4.804 QALYs). In terms of costs, ustekinumab is the most costly treatment (£39,975), followed by adalimumab (£37,852), etanercept (£33,199) and BSC (£21,749). Based on a fully incremental analysis, etanercept is extendedly dominated by adalimumab (i.e. etanercept produces additional gains in effectiveness at incremental costs higher than those of the next most effective strategy of adalimumab, observed by a higher ICER for etanercept than for adalimumab), the ICER of adalimumab compared with BSC is £110,430 per additional QALY and the ICER of ustekinumab compared with adalimumab is £201,507 per additional QALY. The individual pairwise ICERs for etanercept, adalimumab and ustekinumab compared with BSC are £137,059, £110,430 and £116,568 per additional QALY respectively.
There are two important differences to note between the two age populations. First, the reduction in total costs and QALYs for the interventions in the older age group is an artefact of the difference in the model time horizon used in each analysis (i.e. 12 years for age group 6–11 years and 6 years for age group 12–17 years). The time horizon of the model extends until individuals reach 18 years of age, at which point it was assumed that separate NICE recommendations for the interventions in adults apply. A separate scenario analysis is presented below that considers a common time horizon of 14 years for both populations, which is sufficient to capture differences in costs and effects between the interventions. Second, the total costs of etanercept are proportionally greater in the older age group than in the younger age group. This is because of the higher drug acquisition costs of etanercept once individuals reach the age of 10 years, that is, etanercept costs £715 per 4-week cycle in the model (i.e. £178.75 per 50-mg dose each week) for those aged ≥ 10 years and £357.50 per 4-week cycle in the model (i.e. £89.38 per 25-mg dose each week) for those aged < 10 years.
For children and young people aged 6–11 years, adalimumab is the most effective treatment but the incremental gain in QALYs compared with etanercept is relatively small because the utility gains in EQ-5D-Y associated with higher PASI response rates are small. Therefore, the benefits of achieving a greater PASI response do not translate into a large improvement in health outcomes. The benefit of more individuals achieving a higher PASI response rate manifests itself in lower costs associated with less time spent in BSC. The average proportion of individuals achieving a PASI 75 response is 79% for adalimumab and 54% for etanercept. The higher efficacy associated with adalimumab compared with etanercept, which results in fewer individuals accumulating the costs associated with BSC (approximately £284 per 4-week cycle), is not sufficient to offset the additional treatment costs of adalimumab, which are £704.28 per 4-week cycle (i.e. £352.14 per dose every 2 weeks), compared with £357.50 per 4-week cycle for etanercept in children aged < 10 years and £715 per 4-week cycle for children aged ≥ 10 years (note that, although the costs for etanercept increase at age 10 years, there are fewer individuals receiving treatment at this point because the starting age in the model is 6 years and the treatment withdrawal rate is assumed to be 20% per annum).
For children and young people aged 12–17 years, ustekinumab is the most effective treatment but again the incremental gain in QALYs compared with the alternative interventions is relatively small because of the small magnitude of utility gains for the different PASI response categories in the population of children and young people compared with adults (see Utility estimates by health state). The drug acquisition costs of etanercept in young people aged ≥ 12 years are greater than those of adalimumab (£715 for etanercept vs. £704.28 for adalimumab per 4-week cycle) whereas the efficacy for adalimumab is greater than that for etanercept, which reduces the time spent on BSC for those treated with adalimumab. As a result, it might be expected that the total costs of adalimumab would be lower than those for etanercept; however, the improved efficacy of adalimumab also extends the time that individuals receive the intervention and therefore the overall costs of adalimumab increase. Despite this, the incremental costs of etanercept relative to BSC are greater for each additional gain in QALYs than the incremental costs of adalimumab relative to BSC for each QALY gain. As a result, adalimumab extendedly dominates etanercept, which rules out etanercept as a potential cost-effective treatment option.
Treatment with ustekinumab results in the highest average proportion of individuals achieving a PASI 75 response rate (82% vs. 79% for adalimumab and 54% for etanercept), but also has the highest total costs. The higher total costs for ustekinumab compared with adalimumab are the result of the marginally higher drug acquisition costs associated with ustekinumab [£715.67 per 4-week cycle (i.e. £2147.00 per dose with each dose given at 12 weekly intervals) vs. £704.28 per 4-week cycle (i.e. £352.14 per dose at fortnightly intervals) for adalimumab] and the greater cost of ustekinumab during the induction period (i.e. a cost of £2147.00 per dose given at baseline, 4 weeks and 16 weeks) than of adalimumab in the induction period (i.e. a cost of £352.14 per dose given at baseline, 1 week and then every 2 weeks up to week 16). The higher efficacy associated with ustekinumab compared with adalimumab, with an average of 3% more individuals achieving a PASI 75 response, results in a reduction in costs associated with individuals on ustekinumab remaining off BSC for longer, but this reduction is not sufficient to offset the additional treatment costs associated with ustekinumab.
The pairwise ICERs for each of the interventions compared with BSC indicate the ICER at which the particular therapy might enter a sequence. Under base-case assumptions, these ICERs are very high, ranging from £110,430 (adalimumab) to £137,059 (etanercept) per additional QALY in children and young people aged 12–17 years. The optimal treatment option is BSC up until the threshold reaches £111,000 per QALY gained, when adalimumab would then enter as the first treatment in the sequence. The fact that BSC is the only form of management available until the threshold reaches £111,000 per QALY suggests that, under base-case assumptions, none of the biological therapies are sufficiently cost-effective to enter the sequence until this threshold is used. The probability that any of the biologics are cost-effective at a threshold of £30,000 per additional QALY is zero.
Cost-effectiveness results for alternative scenarios
Intervention and comparators
Scenario 1: off-label use of biologics outside age constraints and position in the pathway
As discussed in Decision problem and patient population, the biological interventions differ in their marketing authorisation by age and positioning of treatment in the pathway. Adalimumab is licensed for the youngest age group from ≥ 4 years and is the only biological treatment positioned as a second-line therapy in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies, that is, as an alternative to systemic therapy. This makes the comparison of adalimumab with etanercept and ustekinumab more problematic as the latter interventions are licensed as third-line therapies in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies and who are aged ≥ 6 years in the case of etanercept and ≥ 12 years in the case of ustekinumab. In this scenario, the off-label use of the biologics outside their age constraints and positioning in the management pathway is considered.
In the absence of clinical effectiveness evidence in a systemic therapy-naive population, the same efficacy estimates as in the base-case analysis were used in this scenario. Therefore, the only difference between this scenario and the base-case assumptions is the comparator, which is methotrexate in the analysis that considers biologics as an alternative to systemic therapy, and the time horizon of the model, which extends to 14 years because the starting age in the model is now 4 years.
Table 74 presents the cost-effectiveness results for the use of the interventions as an alternative to systemic therapy for all ages (4–17 years). Ustekinumab is the most effective treatment, followed by adalimumab, etanercept and methotrexate, as the efficacy of the treatments follow in this order. In terms of costs, ustekinumab is the most costly treatment, followed by adalimumab, etanercept and methotrexate. The reason for this ordering is the same as in the base-case results, with ustekinumab costing £715.67 per 4-week cycle compared with a cost per cycle of £704.28 for adalimumab, £357.50 and £715 for etanercept for those aged < 10 years and ≥ 10 years, respectively, and approximately £60 for methotrexate, with the reduction in costs associated with improved efficacy (i.e. less time spent on BSC) not sufficient to offset the additional treatment costs. Based on a fully incremental analysis, the incremental costs of etanercept and adalimumab relative to methotrexate are greater for each additional gain in QALY than the incremental costs of ustekinumab relative to methotrexate for each QALY gain. Therefore, etanercept and adalimumab are extendedly dominated by ustekinumab. The ICER of ustekinumab compared with methotrexate is very high at £293,117 per QALY gained. As a result, the optimal treatment option in a systemic therapy-naive population is methotrexate.
TABLE 74
Scenario 1 results for interventions as an alternative to systemic therapy: off-label use of biologics outside age constraints and position in the pathway
Table 75 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for all ages (4–17 years). The only difference between this scenario and the base-case analysis is the starting age of 4 years used in the model. The total absolute costs and QALYs are greater than in the base case because of the longer model time horizon of 14 years. The ordering of the treatments in terms of costs and QALYs follows that in the base case, with ustekinumab the most effective but most costly treatment, followed by adalimumab, etanercept and BSC. Based on a fully incremental analysis, adalimumab is extendedly dominated by ustekinumab. Compared with the base-case population aged 12–17 years, etanercept is no longer extendedly dominated because more individuals receive a lower dose of etanercept, at the cost of a 25-mg vial rather than a 50-mg vial. The ICERs are lower than in the base-case analysis but the optimal treatment option remains BSC. BSC is the optimal option until the threshold reaches £60,000 per QALY gained, when etanercept would then enter as the first treatment in the sequence.
TABLE 75
Scenario 1 results for treatment with the interventions after failed systemic therapy: off-label use of biologics outside age constraints
Model time horizon
Scenario 2: time horizon of the model
The time horizon of the model was chosen to reflect the fact that once individuals reach 18 years of age separate NICE recommendations for the use of the interventions in adults apply. To incorporate these recommendations, evidence on the efficacy of the treatments in biologic-experienced patients (i.e. effectiveness estimates conditional on previous biological therapy) would be required. This would involve modelling the sequential use of therapies, with every possible potential treatment sequence considered based on current recommendations in adults. As well as being outside the scope of this appraisal, this would represent a significant challenge for two reasons: first, there is very limited evidence on the efficacy of biologics when used in sequence, that is, in biologic-experienced patients, and, second, current NICE recommendations for the use of biologic therapies in moderate to severe psoriasis in adults have been informed by a series of STAs98–102 rather than a MTA that establishes the optimal sequence of treatments in adults.
Furthermore, the differences in the marketing authorisations of the interventions by age inevitably mean that the time horizon of the model will differ according to age group. In this scenario, the impact of the time horizon was assessed by considering a common time horizon of 14 years for all age groups, but with the same starting age for each group as used in the base-case analysis. The time horizon of 14 years is sufficient to capture differences in costs and effects between the interventions under comparison because all individuals on each treatment in the model have moved to BSC by 14 years. This time horizon is also greater than the 10 years used in previous TAs in adults.
The base case already considers a time horizon of 14 years for adalimumab as an alternative to systemic therapy because the starting age is 4 years. Therefore, Table 76 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for a common time horizon of 14 years. By extending the time horizon, the total costs and QALYs for the interventions are greater than in the base case, but the relative cost-effectiveness of the interventions remains the same, that is, the ICERs for each intervention relative to the next best treatment option or BSC are similar to those observed in the base case. Therefore, the model time horizon used in the base-case analysis is sufficient to capture the differences between the interventions in terms of costs and QALYs.
TABLE 76
Scenario 2 results for treatment with the interventions after failed systemic therapy: common time horizon of 14 years
Treatment effectiveness estimates
Scenario 3a: direct trial evidence for treatment effects in children and young people
As discussed in Chapter 4, a NMA was used in the base-case analysis to connect the evidence from the adalimumab trial (M04-717) in children and young people to the evidence from the etanercept (20030211) and ustekinumab (CADMUS) trials by drawing strength from the wider network of evidence in adults. In this scenario, the relative cost-effectiveness of adalimumab compared with methotrexate and of etanercept and ustekinumab compared with BSC is considered using the direct efficacy estimates derived from their corresponding trials. The limitation of this approach is that it does not allow the relative cost-effectiveness of all three biologics to be assessed in the same analysis. However, it may give an indication of how much influence the wider network of evidence has on the individual pairwise comparisons.
Table 77 presents the cost-effectiveness results for use of adalimumab as an alternative to systemic therapy using the efficacy estimates from the M04-717 trial alone. The incremental cost (£20,256) and QALYs (0.037) for adalimumab compared with methotrexate are lower than the base-case incremental cost (£27,084) and QALYs (0.088). The PASI 75 response rate is 58% for adalimumab and 32% for methotrexate in the M04-717 trial compared with 79% and 49%, respectively, in the NMA. The NMA estimates higher absolute values for PASI 75 response but the incremental difference between adalimumab and methotrexate is of a similar magnitude in the NMA (30% difference in PASI 75 response) and the M04-717 trial (26% difference in PASI 75 response). This smaller difference in relative effectiveness between adalimumab and methotrexate in the M04-717 trial means that the incremental cost for each additional gain in QALYs is greater for adalimumab compared with methotrexate. The resulting ICER increases from £308,329 per additional QALY in the base-case analysis to £549,899 per additional QALY using the direct trial evidence.
TABLE 77
Scenario 3a results for adalimumab as an alternative to systemic therapy: direct trial evidence for treatment effects in children and young people
Table 78 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using the efficacy estimates from the etanercept trial (20030211) and the ustekinumab trial (CADMUS). The total costs and QALYs for etanercept and ustekinumab compared with BSC are very similar to those in the base case. This is because the PASI 75 response rates estimated from the NMA for etanercept (54%), ustekinumab (82%) and placebo (11.5%) are very similar to the corresponding response rates from the individual trials (CADMUS: 81% for ustekinumab vs. 11% for placebo; 20030211: 57% for etanercept vs. 11.4% for placebo). As a result, the pairwise ICERs for etanercept and ustekinumab compared with BSC are similar to those in the base-case analysis: the ICER for etanercept compared with BSC increases from £71,903 per QALY in the base-case analysis to £75,350 per QALY using the direct trial evidence and the ICER for ustekinumab compared with BSC increases marginally from £116,568 per QALY in the base-case analysis to £116,982 per QALY using the direct trial evidence.
TABLE 78
Scenario 3a results for treatment with the interventions after failed systemic therapy: direct trial evidence for treatment effects in children and young people
Scenario 3b: indirect treatment comparison estimates in children and young people
In this scenario, the relative cost-effectiveness of etanercept and ustekinumab compared with BSC is considered using the indirect treatment comparison estimates from the 20030211 and CADMUS trials, with placebo used as a common comparator. The limitation of this approach is that it does not allow the relative cost-effectiveness of etanercept and ustekinumab compared with adalimumab to be determined because of the absence of a placebo arm in the M04-717 trial.
Table 79 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using efficacy estimates from an indirect treatment comparison of etanercept from the 20030211 trial and ustekinumab from the CADMUS trial. The total costs and QALYs for etanercept, ustekinumab and BSC are similar to those in the base-case analysis. This is expected as the efficacy estimates from the individual trials for these interventions are similar to those estimated in the NMA. Etanercept is extendedly dominated by ustekinumab as the incremental costs of etanercept relative to BSC are greater for each additional gain in QALYs than the incremental costs of ustekinumab relative to BSC for each QALY gain. This occurs because ustekinumab has a better efficacy (78% PASI 75 response) than etanercept (57% PASI 75 response), which results in improved health outcomes for ustekinumab. Interestingly, the total cost for ustekinumab is greater than that for etanercept despite the fact that the drug acquisition costs are similar between the two treatments in children and young people aged 12–17 years. This arises because, although the improved efficacy of ustekinumab reduces the time spent on BSC, it also means that a greater proportion of time is spent on a cost-ineffective treatment option. The ICER of ustekinumab compared with BSC is £119,092 per QALY gained. As a result, the optimal treatment option is BSC unless the cost-effectiveness threshold reaches £120,000 per additional QALY.
TABLE 79
Scenario 3b results for treatment with the interventions after failed systemic therapy: indirect treatment comparison estimates in children and young people
Scenario 3c: treatment effects from the network meta-analysis using minimum evidence from the adult population
In Chapter 4 the disconnected network of evidence in children and young people was connected in the first instance by bringing together the minimum amount of evidence required from the adult population to link the adalimumab trial with the other paediatric trials. The CHAMPION study in adults,106 which was a three-arm trial comparing adalimumab, methotrexate and placebo, represented the best way of connecting adalimumab to etanercept and ustekinumab using the least amount of evidence borrowed from the adult population. In this scenario, the relative cost-effectiveness of the interventions was considered in the base-case populations using the treatment effects estimated from the minimum network of evidence.
Table 80 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using the NMA with minimum links to the adult evidence. The incremental cost of £18,422 for adalimumab compared with methotrexate is lower than the incremental cost of £27,084 in the base-case analysis. This is because of a larger difference in PASI 75 response rates between adalimumab and methotrexate in the minimum NMA (approximately 40% difference) than in the full network of evidence (approximately 30% difference). Although there is a higher efficacy difference between adalimumab and methotrexate in this scenario, the health outcomes also depend on the utility associated with BSC, which is based on the proportion of individuals in the different PASI response categories in the placebo arm in the NMA. The PASI response rates for placebo are greater in the minimum NMA than in the full network. Therefore, the gain in utility associated with better efficacy on adalimumab is offset by a higher gain in utility associated with BSC. As a result, the incremental QALYs for adalimumab compared with methotrexate are very similar to those in the base-case analysis. The corresponding ICER for adalimumab compared with methotrexate is reduced from £308,329 per additional QALY in the base case to £211,259 per additional QALY.
TABLE 80
Scenario 3c results for adalimumab as an alternative to systemic therapy: treatment effects from the NMA using minimum evidence from the adult population
Table 81 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using the NMA with minimum links to the adult evidence. The incremental costs and QALYs for etanercept and ustekinumab compared with BSC are similar to those in the base-case analysis, but the incremental costs and QALYs for adalimumab are reduced in both age groups. This is because the differences in PASI 75 response rate between the interventions and BSC in the minimum NMA and the full NMA are similar for etanercept (44% vs. 43%) and ustekinumab (66% vs. 71%) but are much smaller for adalimumab (44% vs. 68%). As a result, adalimumab is less cost-effective in children and young people aged 6–11 years (ICER vs. BSC increases from £115,825 per additional QALY in the base case to £137,329 per additional QALY) and is extendedly dominated by ustekinumab in the 12–17 years age group. The ICER for etanercept is reduced by £3400 in children aged 6–11 years, but etanercept is also extendedly dominated by ustekinumab in children aged 12–17 years. The ICER for ustekinumab compared with BSC increases slightly from the base-case value of £116,568 per QALY gained to £118,515 per QALY gained using the minimum NMA.
TABLE 81
Scenario 3c results for treatment with the interventions after failed systemic therapy: treatment effects from the NMA using minimum evidence from the adult population
Scenario 3d: Psoriasis Area and Severity Index response assessment
In this scenario, PASI 50 is considered as the primary efficacy end point for response assessment at the end of the trial period instead of PASI 75, as used in the base-case analysis.
Table 82 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using PASI 50 as the primary efficacy end point. The incremental costs and QALYs for adalimumab compared with methotrexate increase compared with the base case because there is a smaller difference in PASI 50 response rates between the interventions (91.5% adalimumab vs. 71% methotrexate) than for PASI 75 response rates (79% adalimumab vs. 49% methotrexate). As a result, the ICER increases from £308,329 per QALY gained to £353,148 per QALY gained.
TABLE 82
Scenario 3d results for adalimumab as an alternative to systemic therapy: PASI 50 response assessment
Table 83 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using PASI 50 as the primary efficacy end point. The incremental cost per additional QALY gained is greater for all interventions than in the base-case analysis. This is because the total costs have increased (a greater proportion of individuals continue treatment as responders) but the total QALYs have decreased across the interventions. The difference in PASI 50 response rate between the interventions and BSC is similar to the difference observed in PASI 75 response rates. The decrease in QALYs results from the proportionally smaller utility gain associated with the PASI 50–75 response category than with the PASI 75–90 and PASI ≥ 90 response categories. BSC remains the optimal treatment option and the probability that any of the biologics are cost-effective at a threshold of £30,000 per additional QALY is zero.
TABLE 83
Scenario 3d results for treatment with the interventions after failed systemic therapy: PASI 50 response assessment
Health-related quality-of-life utility values
Scenario 4a: EuroQol-5 Dimensions utility estimates from adults
The HRQoL utility values in children and young people are subject to considerable uncertainty. EQ-5D-Y values mapped from PedsQL data from the CADMUS trial (ustekinumab) at baseline and 12 weeks’ follow-up were used to estimate utility gains from baseline associated with different PASI response categories (< 50, 50–74, 75–89, ≥ 90). The utility values associated with treatment were then based on the proportion of individuals in the different PASI response categories from the NMA and the associated utility gain for each PASI category. As discussed in Utility estimates used in the model, the estimated EQ-5D-Y utility gains mapped from the PedsQL data were of a much smaller magnitude than the EQ-5D values used in previous TAs in adults.97–102 It was also noted that the gains in CDLQI score by PASI response category were of a smaller magnitude than the DLQI values reported in adults. It is not clear whether these smaller utility increments observed in children and young people are a reflection of a lower impact of severe psoriasis on quality of life in a paediatric population or a result of the small sample sizes and the limited data in this population.
In this scenario, EQ-5D utility values from the adult population were used to inform the gains in utility associated with PASI response in children and young people. Utility values from TA10397 (etanercept) were used; however, the implications of using alternative adult utility values from TA14699 (adalimumab) and TA180100 (ustekinumab) were also considered (see Table 62 for a comparison of utility values in children and young people and adults).
Table 84 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using utility estimates from an adult population. The total QALYs for the interventions are lower than those in the base-case analysis, but this is because of the use of a lower baseline utility value in this scenario to prevent the utility values rising above 1.0. Note that changing the baseline utility value used in the model does not significantly affect the cost-effectiveness results because the model is driven by the incremental changes in utility from baseline. The incremental QALYs of 0.150 for adalimumab compared with methotrexate are significantly higher than the incremental QALYs of 0.088 in the base case. As a result, the ICER for adalimumab compared with methotrexate reduces from £308,329 to £180,773 per additional QALY. The implications of using adult utility values from TA180100 and TA14699 are even more pronounced, with an incremental gain in QALYs of 0.204 and 0.260, respectively, for adalimumab compared with methotrexate, resulting in corresponding ICERs of £132,616 and £104,010 per additional QALY (see Appendix 8 for results based on utility estimates from TA180100).
TABLE 84
Scenario 4a results for adalimumab as an alternative to systemic therapy: EQ-5D utility estimates from adults
Table 85 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using utility estimates from an adult population. The incremental QALYs for the interventions compared with BSC are substantially greater than those in the base case. Ustekinumab is the most effective intervention, followed by adalimumab, etanercept and BSC, and the incremental gain in QALYs from moving from one intervention to the next is greater than in the base case. As a result, all of the ICERs are substantially lower than in the base case, falling by 55–61%. Etanercept shows the largest reduction in the ICER and, at a threshold of £30,000 per additional QALY, etanercept becomes the optimal treatment in the 6–11 years age group. In the 12–17 years age group, etanercept is extendedly dominated by adalimumab because of the higher drug acquisition costs associated with this age group requiring more than a 25-mg dose. In those aged 12–17 years, the optimal treatment option remains BSC up until a threshold of £51,000 per QALY gained, when adalimumab would then enter as the first treatment in the sequence. At a threshold of £60,000 per QALY, adalimumab represents the only cost-effective treatment option based on a fully incremental analysis, whereas all of the biologics would be considered cost-effective based on a pairwise comparison with BSC.
TABLE 85
Scenario 4a results for treatment with the interventions after failed systemic therapy: EQ-5D utility estimates from adults
The implications of using adult utility values from TA180100 and TA14699 are even more pronounced than when using utility gains from TA10397 because of the greater utility gains in the PASI 75–89 and ≥ 90 categories. The ICERs for children and young people aged 6–11 years are £22,578 (TA14699) and £21,546 (TA180100) for etanercept compared with BSC and £37,125 (TA14699) and £39,682 (TA180100) for adalimumab compared with BSC. The lowest ICERs for children and young people aged 12–17 years are £33,517 for adalimumab compared with BSC, £35,612 for ustekinumab compared with BSC and £39,247 for etanercept compared with BSC.
Scenario 4b: utility estimates for best supportive care
The base-case analysis assumes that the utility associated with BSC is based on the proportion of individuals in the different PASI response categories in the placebo arm of the NMA. In this scenario, the utility for BSC was set equal to the baseline value, that is, assuming that there is no utility gain associated with BSC.
Table 86 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy assuming that there is no utility benefit associated with BSC. For the comparison of adalimumab and methotrexate, the assumption of no utility benefit on BSC affects only the utility of non-responders. The total QALYs for both interventions are reduced and the incremental QALYs for adalimumab compared with methotrexate increase from 0.088 in the base case to 0.102 because of the higher efficacy of adalimumab, which reduces the time spent in BSC.
TABLE 86
Scenario 4b results for adalimumab as an alternative to systemic therapy: utility in BSC equal to the baseline value
Table 87 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy assuming that there is no utility benefit associated with BSC. This assumption reduces the total QALYs for the comparator of BSC and the utility of non-responders. As a result, the incremental QALYs for the interventions compared with BSC increase and the incremental gain in QALYs for the interventions relative to the next best option (e.g. ustekinumab is the most effective treatment, followed by adalimumab and etanercept) also increases as less time is spent on BSC. Consequently, the ICERs for the interventions are reduced compared with the base-case values.
TABLE 87
Scenario 4b results for treatment with the interventions after failed systemic therapy: utility in BSC equal to the baseline value
Costs associated with best supportive care
Scenario 5a: number of hospitalisations per annum for best supportive care
The resource use associated with BSC, in particular the number of hospitalisations per annum, was identified as an area of high uncertainty and a key driver of cost-effectiveness in previous TAs in adults.97–102 Two main sources have been referred to in previous appraisals: (1) NICE CG153,173 in which an average of 26.6 inpatient days per year was estimated for individuals whose psoriasis has not responded to treatment, and (2) Fonia et al.,144 who estimated an average of 6.49 days of hospitalisation per annum. During previous NICE appraisals, the clinical experts considered that both sources are likely to overestimate the actual number of hospital days and resource use associated with BSC. This is in part because of the populations considered in CG153173 and the study by Fonia et al.,144 with CG153173 considering a high-need population with very severe psoriasis and the study by Fonia et al.144 describing care in a tertiary care centre known for treating the most severely affected individuals. The clinical experts in recent appraisals also noted that the number of individuals hospitalised for severe psoriasis has fallen over time and is continuing to fall. They also indicated that BSC is mostly given to individuals during their outpatient visits. As a result, the resource use associated with BSC is an area of considerable uncertainty and both sources of data have a number of shortcomings, even in the adult population.
In the base-case analysis in children and young people, it was assumed that there are no hospitalisations for psoriasis in this population. This was informed by clinical opinion, with our clinical advisor suggesting that hospitalisations in children and young people are very rare, partly because this population has not yet developed the comorbidities that often complicate more severe cases of psoriasis in adults. In this scenario, the implications of assuming no inpatient stays for children and young people were explored by using an estimate of 6.49 hospitalisations per annum based on the study by Fonia et al.144 and 26.6 hospitalisations per annum based on CG153173 in adults.
Table 88 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy, assuming hospitalisations for BSC. For the comparison of adalimumab and methotrexate, the total costs for both interventions are increased, but the incremental cost of adalimumab compared with methotrexate decreases because of the higher efficacy associated with adalimumab, which reduces the time spent in BSC. The resulting ICER decreases from £308,329 per additional QALY in the base case to £281,029 per additional QALY for 6.49 inpatient days per annum and £202,571 per additional QALY for 26.6 inpatient days per annum.
TABLE 88
Scenario 5a results for adalimumab as an alternative to systemic therapy: number of hospitalisations per annum for BSC
Table 89 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy, assuming hospitalisations for BSC. Under this assumption, the costs of BSC increase by £147.67 and £605.25 per 4-week cycle for 6.49 and 26.6 inpatient days per annum respectively. As a result, the total costs associated with the comparator of BSC increase and the costs for non-responders increase. For children and young people aged 6–11 years, the reduction in the incremental cost of etanercept compared with BSC is sufficient to make etanercept the optimal treatment option at a threshold of £30,000 per QALY for a stay of 6.49 inpatient days per annum. When the hospitalisation LOS per annum is increased to 26.6 days in this age group, etanercept becomes the least costly treatment option and BSC becomes dominated by etanercept (i.e. BSC costs more than etanercept but produces fewer QALYs). Adalimumab enters as a cost-effective option only if the threshold increases to £70,000 per QALY gained.
TABLE 89
Scenario 5a results for treatment with the interventions after failed systemic therapy: number of hospitalisations per annum for BSC
For children and young people aged 12–17 years, etanercept is dominated by adalimumab. The ICER for adalimumab compared with BSC is £74,501 per QALY when 6.49 inpatient days per annum are assumed. When 26.6 inpatient days per annum are assumed, adalimumab becomes the least costly treatment option and the most cost-effective option at a threshold of £30,000 per QALY. The ICER for ustekinumab compared with adalimumab is £118,665 per QALY for a stay of 26.6 inpatient days per annum.
Costs associated with adverse events
Scenario 6: costs of severe infections and malignancies
In the absence of robust evidence on the incidence of AEs associated with treatment in children and young people, the base-case analysis assumed that there were no AEs associated with treatment. In this scenario, the costs associated with SAEs, including NMSC, malignancies other than NMSC and severe infections, are included. These events are expected to be very rare.
Table 90 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy with the costs of AEs included. The incremental cost of adalimumab increases by only £400. The resulting impact on the ICER is minor, with an increase from £308,329 per QALY gained in the base case to £311,067 per QALY gained.
TABLE 90
Scenario 6 results for adalimumab as an alternative to systemic therapy: costs of severe infections and malignancies included
Table 91 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy with the costs of AEs included. As expected, the incremental costs for the interventions relative to BSC increase, but the resulting impact on the ICERs for all interventions is very minor.
TABLE 91
Scenario 6 results for treatment with the interventions after failed systemic therapy: costs of severe infections and malignancies included
Treatment withdrawal rates
Scenario 7: withdrawal rates from treatment
In the base-case analysis discontinuation from treatment is modelled as an all-cause withdrawal probability of 20% per annum, which is applied to all interventions. This withdrawal rate has been used in all previous TAs in adults97–102 and is consistent with long-term survival rates of biologics from the BADBIR audit.94 In the absence of alternative data in children and young people, this scenario considered two separate withdrawal rates of 10% and 30% per annum.
Table 92 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy for treatment withdrawal rates of 10% and 30% per annum. The lower withdrawal rate implies that individuals spend longer on treatment before moving to BSC, whereas the higher withdrawal rate means that individuals spend less time on treatment and more time on BSC. The total costs for adalimumab increase for the 10% rate and decrease for the 30% rate, whereas the total costs for methotrexate decrease for the 10% rate and increase for the 30% rate. This opposite effect between the treatments arises because the drug acquisition cost of adalimumab (£704.28 per 4-week cycle) is proportionally greater than the cost of BSC (approximately £284 per 4-week cycle) compared with the drug acquisition cost of methotrexate (approximately £60 per 4-week cycle) relative to the cost of BSC. As a result, the withdrawal rate has less impact on the total cost of methotrexate than on the total cost of adalimumab. The incremental costs of adalimumab compared with methotrexate are £40,781 and £19,692 for the 10% and 30% annual withdrawal rates, respectively, compared with the base-case incremental cost of £27,084. In terms of health outcomes, the more time spent on treatment the higher the utility gains; therefore, the QALYs increase for the lower withdrawal rate and decrease for the higher withdrawal rate. The resulting ICERs for adalimumab compared with methotrexate are £298,846 and £318,188 per additional QALY for the 10% and 30% annual withdrawal rates, respectively, compared with the base-case value of £308,329 per additional QALY.
TABLE 92
Scenario 7 results for adalimumab as an alternative to systemic therapy: treatment withdrawal rates of 10% and 30% per annum
Table 93 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for treatment withdrawal rates of 10% and 30% per annum. The total costs for all of the interventions increase for the 10% rate and decrease for the 30% rate because of the accumulation of higher drug acquisition costs while on treatment for longer. This increase in costs is counterbalanced by an increase in utility gains while on treatment. The resulting impact on the ICERs is minimal. BSC remains the optimal treatment option at a threshold of £30,000 per QALY gained.
TABLE 93
Scenario 7 results for treatment with the interventions after failed systemic therapy: treatment withdrawal rates of 10% and 30% per annum
Biosimilars
Scenario 8: reduction in the cost of etanercept
The biosimilar of etanercept, Benepali (50 mg), does not have marketing authorisation for use in children and young people. In this scenario, the drug acquisition cost of etanercept was reduced by approximately 10% to match the cost of Benepali in adults.
Table 94 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for a 10% reduction in the acquisition cost of etanercept. For children and young people aged 6–11 years, the incremental cost of etanercept relative to BSC is reduced by £580, which reduces the ICER from £71,903 to £66,240 per additional QALY. For children and young people aged 12–17 years, the incremental cost of etanercept relative to BSC is reduced by £1480, which is a greater reduction than that observed in the younger age group because it is assumed that the 10% reduction in the drug acquisition cost of etanercept applies only to children aged ≥ 10 years who require 50 mg of etanercept. The cost reduction has a very minor impact on the cost-effectiveness results.
TABLE 94
Scenario 8 results for treatment with the interventions after failed systemic therapy: reduction in the cost of etanercept to match the unit cost of Benepali
Discussion of the cost-effectiveness results and alternative scenarios
The results of the base-case analysis suggest that adalimumab is not a cost-effective treatment option when positioned in the pathway as an alternative to systemic therapy, with an ICER of £308,329 per QALY gained compared with methotrexate. When positioned after systemic therapy, the ICER for adalimumab compared with BSC is £115,825 per QALY gained for ages 6–11 years and £110,430 per QALY gained for ages 12–17 years. At a threshold of £30,000 per QALY gained, etanercept is not a cost-effective option for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies. The ICER for etanercept compared with BSC is £71,903 per QALY gained for ages 6–11 years and etanercept is extendedly dominated by adalimumab for those aged 12–17 years. Ustekinumab is the most effective treatment in children and young people aged 12–17 years but it is also the most costly treatment. Based on a fully incremental analysis, the ICER for ustekinumab compared with adalimumab is £201,507 per QALY gained, whereas the ICER for ustekinumab compared with BSC is £116,568 per QALY gained. The base-case results suggest that BSC is the only cost-effective form of management for the treatment of severe plaque psoriasis unless the threshold reaches ≥ £111,000 per additional QALY. The probability that any of the biologics are cost-effective at a threshold of £30,000 per QALY is zero.
The lack of cost-effectiveness appears to result from the very modest QALY gains associated with treatment. The small incremental difference in health benefits between the treatments is a result of the relatively small EQ-5D-Y utility gains associated with higher PASI response rates. As a consequence, the benefits of achieving a greater PASI response do not translate into large improvements in health outcomes. The acquisition costs of the treatments are also not substantially different: ustekinumab costs £715.67 per 4-week cycle (i.e. £2147.00 per dose with each dose given at 12 weekly intervals) compared with £704.28 per 4-week cycle (i.e. £352.14 per dose given every 2 weeks) for adalimumab and £715.00/£357.50 per 4-week cycle (i.e. £178.75 per 50-mg/£89.38 per 25-mg dose given each week) for etanercept, depending on patient weight.
A number of scenarios were used to explore the impact of alternative assumptions on the cost-effectiveness of the biological treatments. Tables 95 and 96 summarise the cost-effectiveness results for the scenario analyses for adalimumab as an alternative to systemic therapy and the use of the interventions after failed systemic therapy respectively.
TABLE 95
Summary of the cost-effectiveness results for adalimumab as an alternative to systemic therapy: base-case results and alternative scenarios
TABLE 96
Summary of the pairwise cost-effectiveness results for treatment with the interventions after failed systemic therapy: base-case results and alternative scenarios
The scenarios that have the most impact on the cost-effectiveness results are (1) use of utility estimates from an adult population (scenario 4a), (2) assuming that no health benefits are associated with BSC (scenario 4b) and (3) assuming that hospitalisations are associated with BSC (scenario 5).
The gains in utility in the adult population for the different PASI response categories are up to 6.6 times greater than the utility gains estimated in children and young people. It is unclear whether this difference reflects a lower impact of severe psoriasis on HRQoL in children and young people or the limited data available in this population and the significant uncertainty surrounding quality-of-life estimates in paediatric psoriasis populations. The use of utility values from an adult population brings the ICER for etanercept compared with BSC under the threshold of £30,000 per QALY gained in children and young people aged 6–11 years. The ICERs for ustekinumab and adalimumab using adult utility data are reduced significantly but remain above the £30,000 per QALY threshold, even using the most favourable estimates from TA146.99 Under the assumption of no health benefits associated with BSC, the ICERs are reduced by up to £30,000 from the base-case values but remain quite high, with the lowest ICER of £56,430 per QALY gained for etanercept compared with BSC.
The number of hospitalisations associated with BSC is a key driver of the cost-effectiveness of the biological interventions. This was also identified as a key consideration in previous TAs in adults.97–102 Based on clinical opinion, in the base-case analysis it was assumed that hospitalisations for severe psoriasis are very rare in children and young people. If the average hospitalisation LOS per annum is increased to 6.49 days based on the study by Fonia et al.,144 the ICERs for the interventions reduce significantly; however, the only ICER that falls below the threshold of £30,000 is for the use of etanercept compared with BSC in children and young people aged 6–11 years. If the average number of hospitalisations per annum is increased significantly to 26.6 days per annum, based on the very high-need population described in CG153,173 the biological treatments compared with BSC are all considered cost-effective in individuals who have failed systemic therapy. However, recent appraisals in adults have considered the estimate of 26.6 days per annum to be too high.
The combined impact of the most optimistic utility estimates in adults (TA14699), 6.49 inpatient days per annum and no health benefits for BSC are presented in Tables 97 and 98 for the use of the interventions before and after systemic therapy. The combined impact of the utility gains from an adult population and an assumption of 6.49 hospitalisations per annum is sufficient to reduce the pairwise ICERs for the interventions compared with BSC to below a threshold of £30,000 per additional QALY, whereas the additional assumption of no health benefits for BSC reduces the ICERs further to below a threshold of £20,000 per additional QALY. Based on a fully incremental analysis, etanercept is the optimal treatment for children and young people aged 6–11 years, whereas adalimumab is the optimal treatment for children and young people aged 12–17 years.
TABLE 97
Combined impact of alternative assumptions on the cost-effectiveness of adalimumab as an alternative to systemic therapy
TABLE 98
Combined impact of alternative assumptions on the cost-effectiveness of the interventions after failed systemic therapy
- Independent economic assessment - Adalimumab, etanercept and ustekinumab for tre...Independent economic assessment - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...
