NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Duarte A, Mebrahtu T, Goncalves PS, et al. Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2017 Nov. (Health Technology Assessment, No. 21.64.)

Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation.
Show detailsOverview
Randomised controlled trials of the effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people have been discussed and summarised in Chapter 3. The efficacy end point consistently reported across the trials was PASI response rates, which is the key efficacy parameter used in the economic analysis. To determine the relative efficacy of the interventions, it would be ideal to have results from good-quality adequately powered RCTs comparing the active treatments with one another in the population of children and young people. However, the evidence base presents a number of challenges for informing the relative efficacy of the interventions in this population. First, the interventions of interest have not been directly compared in head-to-head RCTs. Second, no common comparator (e.g. placebo) exists across all of the RCTs. Third, the age of the populations included in the trials differs across the RCTs and the interventions of interest have marketing authorisation for different age groups. Fourth, the severity of plaque psoriasis is defined differently in the populations included in the RCTs and the interventions are licensed for different levels of psoriasis severity in children and young people. These challenges mean that a number of assumptions are required to inform the benefits of the active treatments relative to the appropriate comparators and each other.
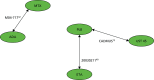
Meta-analysis using mixed-treatment comparisons enables the estimation of different parameters from several studies with similar comparisons to be combined when direct evidence on comparisons of interest is absent or sparse. The statistical synthesis method of NMA enables the comparison of multiple treatment options using both direct comparisons of interventions from RCTs and indirect comparisons across trials based on a common comparator.95,96 As suggested by the term, NMA needs a ‘network of evidence’ to be established between all of the interventions of interest. However, with neither direct comparisons nor a common comparator in the evidence base for children and young people from which to derive indirect comparisons of comparator treatments, the evidence base is structured as a ‘disconnected network’ (Figure 3).
In the following sections we build on the challenges listed above by exploring treatment efficacy by age subgroup and by performing a naive indirect treatment comparison of adalimumab and etanercept, highlighting the limitations of such analysis. Furthermore, a framework of analysis is described that uses different levels of evidence from the adult population to specifically address the issue of having a disconnected network structure.
Efficacy differences by age subgroup
Adalimumab, etanercept and ustekinumab have marketing authorisation for different age groups in the population of children and young people (≥ 4 years for adalimumab, ≥ 6 years for etanercept and ≥ 12 years for ustekinumab). This is the result of variation in the ages of the patient populations included in the RCTs for these interventions. Furthermore, the trial population for etanercept also included patients who were younger than the licensed age group (i.e. the inclusion criteria for the etanercept trial was children and adolescents aged 4–17 years and nine children were included in the trial who were younger than the subsequent licensed age group of ≥ 6 years). To establish the relative efficacy of the interventions it was necessary to either (1) assume that the PASI response rates for the treatments are independent of age within the full population of children and young people or (2) consider outcomes in a population subgroup by age.
Chapter 3 presents the PASI response rates for each study by age subgroup. On inspection of the PASI response rates, there does not appear to be a pattern across the efficacy outcomes for the different age subgroups within the same study, which could explain any differences in efficacy as a result of age. This would seem to suggest that the PASI response rates for the study as a whole are reflective of the outcomes expected in a particular subpopulation by age. This was examined further by using standard parametric statistical tests to assess the equality of proportions (i.e. the probability of PASI 50/75/90 response rates) across different age subgroups within each study. Within each study there were no statistically significant differences identified across the age subgroups for each of the PASI response rates of 50, 75 or 90 (Table 36). Therefore, to compare the relative efficacy of the interventions, it was assumed that the PASI response rates for the treatments are independent of age within the full population of children and young people and that the studies are comparable for this population.
TABLE 36
Hypothesis testing of age subgroup PASI responses by study and treatment arm
Indirect treatment comparison
Figure 3 shows that there is no common comparator arm between the adalimumab trial (M04-717) and the trials of etanercept (20030211) and ustekinumab (CADMUS), with the adalimumab trial having a comparator of methotrexate and the other two trials having a comparator of placebo. Therefore, it is not possible to establish an indirect comparison between adalimumab and etanercept or ustekinumab without drawing on evidence from other sources (e.g. evidence on the relative efficacy of the interventions in adults) or by creating a common comparator (e.g. assuming that the methotrexate and placebo response rates are exchangeable between the trials). In this section, attention is focused on the indirect comparison that can be established between etanercept and ustekinumab.
An indirect treatment comparison of PASI response rates at 12 weeks was performed between the licensed doses of etanercept (0.8 mg/kg up to a maximum dose of 50 mg) and ustekinumab (standard dose) using placebo as a common comparator. A Bayesian indirect treatment comparison was undertaken using a probit model for ordered multinomial outcomes of PASI response rates (using a fixed-effect model with multinomial likelihood and a probit link (see Appendix 4). Table 37 presents the absolute probabilities of PASI 50, 75 and 90 responses for etanercept and ustekinumab and Table 38 presents the relative treatment effects expressed as RRs with 95% Bayesian credible intervals (CrIs).
TABLE 37
Absolute probabilities of PASI 50, 75 and 90 response rates in the indirect treatment comparison of etanercept and ustekinumab
TABLE 38
Relative effect estimates (as RRs, means and 95% CrIs) for each treatment combination for PASI 75 in the direct trial comparisons (upper diagonal) and the indirect treatment comparison for etanercept and ustekinumab (lower diagonal)
The results demonstrate that ustekinumab appears to be more effective than etanercept in this population. The PASI 75 absolute probability of response for ustekinumab at 12 weeks was estimated to be 78% (95% CrI 63% to 90%) whereas that for etanercept was estimated to be 57% (95% CrI 44% to 69%). The 95% CrIs were wide and overlap, which reflects the small sample size and limited number of data points used in this analysis. The pooled RR presented in Table 38 for ustekinumab compared with etanercept is 1.41, but this is not statistically significant as the 95% CrI includes 1. The indirect comparison results are in line with the direct evidence from the clinical trials.
The company submission for ustekinumab presented a similar indirect treatment comparison for ustekinumab compared with etanercept [Janssen. Adalimumab, Etanercept and Ustekinumab for Treating Plaque Psoriasisin Children and Young People – Company Evidence Submission. ID854. 2016 (unpubished)]. The company’s analysis produced results for the full population and for a subgroup aged 12–17 years. The results of the company’s full population NMA are broadly similar to the results from the Assessment Group’s (AG) analysis, for example the ustekinumab PASI 75 response was estimated to be 79.8%, compared with 78.1% in Table 37.
It is important to note that these analyses are limited for a number of reasons.
- It draws conclusions only regarding the short-term use of ustekinumab and etanercept in the population of children and young people from the corresponding trials.
- The placebo arms in the etanercept trial (20030211) and the ustekinumab trial (CADMUS) are assumed to be exchangeable between the trials.
- Inclusion criteria for age were different between the trials.
- There is uncertainty in both the within-trial and between-trial treatment effect estimates because of the small sample sizes in the trials.
- There are differences between the trials in terms of baseline characteristics and trial design: these differences have been explored separately in Chapter 3.
- The indirect treatment comparison does not provide sufficient information to inform the economic analysis as the use of adalimumab has been excluded from the analysis because of a lack of a common comparator.
As a consequence of the above limitations, in particular the exclusion of the adalimumab trial (M04-717) evidence, the results in Table 37 could not be used to assess the relative cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people.
Framework of analysis for informing the relative efficacy of the interventions
Because of the lack of a common comparator arm between the adalimumab trial (M04–717) and the ustekinumab (CADMUS) and etanercept (20030211) trials, an analysis plan was developed that entailed exploring the possibility of using PASI response data from adults with moderate to severe plaque psoriasis to fill the evidence gap in the population of children and young people. The use of data from the adult population was supported by our clinical advisor (Dr Ruth Murphy, personal communication), who did not see any reason why the relative effectiveness of the interventions in adults could not be used to infer relative effectiveness in children and young people, especially in the absence of evidence in the latter population.
A framework of analysis was thus developed for the NMA approach that allowed all available and relevant evidence to be included. The framework explored two separate networks, which differed according to the extent of evidence utilised from the adult trials.
- The network of trials in children and young people was connected by bringing the minimum amount of evidence required from the adult population to link the adalimumab trial with the other trials in the disconnected network in Figure 3.
- The network of trials in children and young people was connected by bringing together all relevant evidence on the efficacy of all of the interventions in adults.
This approach allows treatment-specific estimates to be modelled in each population by drawing strength from the network of evidence available. The use of a NMA in preference to pairwise meta-analyses enables the inclusion of all relevant evidence, allowing for precise estimates of treatment effects to be calculated. In addition, the results from the NMA feed directly into the economic model to provide the relevant cost-effectiveness of adalimumab, etanercept and ustekinumab compared with relevant comparators and each other. This approach has been used in previous NICE TAs for the treatment of plaque psoriasis in adults (TA103,97 TA134,98 TA146,99 TA180,100 TA350,101 and TA368102).
In each of the NICE TAs in adults the evidence network was updated with new studies reported since the previous appraisal. Therefore, we took the most recent single TAs in adults (TA368102 and TA350101) as the starting point for developing a network of studies that could potentially connect the adalimumab trial in children and young people to the other interventions. The Evidence Review Groups (ERGs) for these appraisals generally rated the systematic reviews underpinning the identification of trials for inclusion in the NMA as appropriate and the evidence networks were subsequently used to inform NICE recommendations in these appraisals. Therefore, it was assumed that the vast majority of relevant evidence for the interventions in adults had been captured in the most recent appraisals in 2015.101,102 Relevant adult trials were identified based on the indirect comparison and/or multiple treatment comparisons reported within these appraisals. Lists of excluded trials and reasons for exclusion were also reviewed and relevant trials identified. To supplement this review, the results of a recently published systematic review and NMA,103 which adjusted for cross-trial differences in the comparative efficacy of biological treatments for moderate to severe psoriasis in adults, were also examined to cross-check that the majority of relevant studies had been identified in the previous appraisals. Furthermore, we also considered studies reported in the original multiple TA in adults (TA10397), which included interventions such as methotrexate and ciclosporin. The key inclusion and exclusion criteria used to identify relevant trials for the NMA are shown in Table 39. A list of excluded trials (n = 18) and reasons for exclusion can be found in Appendix 5. Table 40 presents a summary of the trials in adults, including the comparator agents used in each trial, which was used to inform the NMA.
TABLE 39
Key inclusion and exclusion criteria for adult studies
TABLE 40
Summary of trials in adults selected to inform the NMA
Thirty-four trials in adults with moderate to severe plaque psoriasis were found to be relevant for the NMA; 29 of these considered a placebo arm and six were three-arm trials. As described in the ERG reports for the previous TAs, selected studies were mostly comparable in terms of their inclusion criteria regarding previous and concomitant medication use. The majority of studies included patients who had failed or who had had an insufficient response to previous topical therapy and conventional systemic agents such as ciclosporin or methotrexate. Some studies included only biologic-naive individuals, whereas others allowed previous biological therapy use. Almost all of the studies did not allow concomitant treatment with systemic agents or phototherapy. A few studies did not mention their criteria regarding concomitant medication use.
The full set of interventions and comparators included adalimumab, etanercept, 45 mg of ustekinumab, 90 mg of ustekinumab, apremilast, methotrexate, ciclosporin, fumaric acid, infliximab and placebo. Response rates for PASI 50, 75 and 90 from the selected trials were identified and extracted, together with sample size and key baseline patient characteristics by treatment arm. Table 41 presents a summary of the data extracted together with the corresponding data from the three trials in children and young people.
TABLE 41
Summary of PASI response data used in the NMA and key baseline patient characteristics by treatment arm
Network meta-analysis using minimum evidence from the adult population
The disconnected network of evidence in children and young people was connected in the first instance by bringing together the minimum amount of evidence required from the adult population to link the adalimumab trial with the other trials (Figure 4). Among the studies presented in Table 40, there was only one trial in adults that could directly connect methotrexate with placebo and adalimumab with placebo (CHAMPION106). A number of trials compared adalimumab with placebo alone but inclusion of these trials would mean that methotrexate was connected only indirectly through adalimumab and placebo, potentially undermining the evidence from M04-717 on this agent. Therefore, the CHAMPION study represented the best way to connect adalimumab and methotrexate to etanercept and ustekinumab using the least amount of evidence drawn from the adult population.
In the CHAMPION trial,106 the primary efficacy end point was the proportion of individuals achieving a PASI 75 response at 16 weeks. Adalimumab was found to have significantly greater efficacy (79.6% achieving a PASI 75 response) than either methotrexate (35.5%) or placebo (18.9%). PASI outcome data and key baseline characteristics for the CHAMPION trial are provided in Table 41. The average age of patients recruited into the CHAMPION trial was approximately 42 years. The CHAMPION trial was a larger trial than the trials carried out in children and young people (n = 271 vs. n = 75 in the M04-717 trial), with an approximately 10–20% higher proportion of males.
The PASI 75 response rates for adalimumab and methotrexate in the CHAMPION trial were similar to those reported in the M04-717 trial in children and young people. An important difference between the CHAMPION trial and the trials in children and young people was the observed placebo effect on the primary end point of PASI 75. Whereas in the etanercept trial (20030211) and the ustekinumab trial (CADMUS) the proportion of individuals achieving a PASI 75 response in the placebo arm was approximately 11%, the proportion achieving a PASI 75 response in the placebo arm in the CHAMPION trial106 was approximately 19%. The authors of the CHAMPION trial identified two reasons for this anomalous placebo response: (1) placebo response rates are generally greater in European studies and (2) the observed placebo response may partly have resulted from the correction of an underlying folate deficiency following folate supplementation, which was mandatory for all study patients.
Given that the CHAMPION trial connects the adalimumab trial in children and young people (M04-717) to etanercept and ustekinumab through placebo, it is important to ensure that the differences in placebo response rates do not ‘artificially’ inflate or deflate the PASI response outcomes for the interventions of interest. Therefore, as well as using a baseline unconstrained prediction model, whereby baseline risk (placebo response rates) is predicted using evidence from all studies included in the network (analysis 1a), a baseline constrained prediction model was also considered, whereby placebo response rates are predicted based on the placebo arm trials in children and young people only [i.e. the etanercept (20030211) and ustekinumab (CADMUS) trials] (analysis 1b). As the number of trials to inform each treatment effect is small, a fixed-effect model was used. The results of this analysis are presented in Results.
Network meta-analysis using full evidence from the adult population
The second approach to the NMA involved connecting the evidence from the adalimumab trial in children and young people to the evidence from the other trials (20030211 and CADMUS) by drawing strength from the full network of evidence available in adults. The relative efficacy of adalimumab, etanercept and ustekinumab has been evaluated extensively in adults with moderate to severe plaque psoriasis. Given the limited evidence base in children and young people, and the expectation that the difference in response rates between the interventions is predominantly the result of the relative efficacy of the biologics rather than age or other patient characteristics, it would seem appropriate to combine the weight of evidence from all relevant trials and comparators, including those in adults. This wider network of evidence can be used to facilitate an indirect comparison of adalimumab with etanercept and ustekinumab by examining the relationships that exist between the different treatments and study populations and drawing strength from the full network of evidence.
Figure 5 presents the full network of evidence in both populations. This wider network considers nine active treatments and placebo, encompassing 37 RCTs in total (three in children and young people and 34 in adults), with six of these being three-arm trials. The majority of network links (‘head-to-head trial comparisons’) are populated by more than one study.
A Bayesian evidence synthesis approach was employed that draws on the relationships that exist between treatments and populations while also preserving differences that exist across populations by adjusting for age and placebo response rates. NMA meta-regression models on baseline risk (i.e. placebo response) were explored.103 These models impose a common interaction effect between baseline risk and relative effectiveness that accounts for variation in reference arm response across trials. NMA meta-regression models that explore variability caused by age effects were also implemented. These models impose an age group interaction effect at the study level (binary variable: 1 if study is from a child or young adult population, 0 otherwise) that attempts to explain the heterogeneity between treatment effects when considering both adult treatment response data and data from children and young people. The age-adjusted meta-regression models provided pooled PASI response rates by treatment for both children and young people, and adults. A common treatment × age interaction effect was imposed. The common interaction assumption is the least data demanding (i.e. only one extra parameter needs to be estimated), but it also imposes the strongest assumption as it implies that the same age group effect exists regardless of treatment (excluding placebo).138 For example, if the age interaction effect (of children and young people vs. adults) is estimated to be positive and of average magnitude 25% on the absolute PASI scale, PASI response rates in children and young people will be approximately 25% higher, on average, than those in adults, irrespective of treatment. Further details on the implemented synthesis models and their assumptions, including the WinBUGS code (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK), are provided in Appendix 5.
Fixed- and random-effects analyses were explored for two separate scenarios: (1) a meta-regression model with adjustment for baseline risk (i.e. placebo response rates) and (2) a meta-regression model with adjustment for baseline risk and age. Irrespective of scenario and according to deviance information criterion (DIC) and total residual deviance statistics, the random-effects approach provided a better fit to the data than the fixed-effect counterpart. Therefore, only results from the random-effects model are presented and discussed here. The results from the fixed-effect model are provided in Appendix 6.
Table 42 provides a summary of the models implemented together with the key modelling assumptions. As no evidence was found to support the existence of a class effect, all models considered treatments to be independent of each other. In models in analyses 2a and 2b it was assumed that treatments were independent of each other, but treatment effects were adjusted with the trial-specific baseline effects, assuming a common interaction term. In addition, models in analysis 2b were adjusted for trial-specific age effects, also assuming a common interaction term. This age adjustment enabled the estimation of treatment effects separately by age (adults and children and young people). All implemented synthesis models assumed fixed effects on PASI response cut-off points.
TABLE 42
Summary of models implemented and key modelling assumptions
Results
Analysis 1: results using minimum evidence from the adult population
Table 43 summarises the results of the NMA in terms of absolute PASI response rates for the unconstrained (no explicit adjustment for differences in placebo response rates across the trials) and constrained (placebo response rates predicted based on the placebo arm trials in children and young people only) models. The results of both sets of analyses show that all active treatments are more effective than placebo. In terms of mean response rates (analysis 1b results), ustekinumab is estimated to have the highest probability of achieving a PASI 50 (90%, 95% CrI 81% to 96%), PASI 75 (79%, 95% CrI 64% to 90%) and PASI 90 (57%, 95% CrI 39% to 74%) response compared with any of the other treatments, suggesting that it is the most effective intervention. This is followed by adalimumab, etanercept and methotrexate in both sets of analyses, that is, the ranking of treatments based on mean response rates is unchanged in the different models.
TABLE 43
Network meta-analysis results for analyses 1a and 1b: probability of achieving PASI 50, 75 and 90 response rates
The unconstrained baseline model (analysis 1a), however, predicts a placebo effect for PASI 75 of 20.3% (95% CrI 14% to 27%) whereas the constrained baseline model (analysis 1b) predicts a placebo effect of 13.1% (95% CrI 8% to 19%). This difference is driven by the CHAMPION trial,106 which had a substantially higher placebo response rate of approximately 19% for PASI 75 compared with the placebo response rates observed in the trials of children and young people (approximately 11% in 20030211 and CADMUS). The constrained baseline model (analysis 1b) adjusts the baseline predictions to consider only placebo effect evidence from trials in the younger population. In this analysis, the mean PASI 75 response rate for placebo is reduced and closer to the observed response in the children and young people trials.
As shown by the CrIs around the mean response rates, which are wide and overlap, there is uncertainty around these response rates. This is also shown in terms of the RRs of each treatment compared with placebo and their CrIs for the best-fitting model 1b (Table 44).
TABLE 44
Relative effect estimates (as RRs, means and 95% CrIs) for each treatment combination for PASI 75 in the direct trial comparisons (upper diagonal) and from the NMA results for analysis 1b (lower diagonal)
Analysis 2: results using all relevant evidence from the adult population
Table 45 summarises the absolute PASI response rates from the NMA that uses the full network of evidence in both populations for the unadjusted random-effects model (analysis 2). Relative treatment effects for analysis 2 for PASI 75 response are presented in Table 46. The random-effects approach outperformed the fixed-effect approach in terms of model fit, suggesting that accounting for between-study heterogeneity is an important factor (τ2 = 0.02).
TABLE 45
Network meta-analysis results for analysis 2: probability of achieving PASI 50, 75 and 90 response rates
TABLE 46
Relative effect estimates (as RRs, means and 95% CrIs) for each treatment combination for PASI 75 in the direct trial comparisons (upper diagonal) and from the NMA results for analysis 2 (lower diagonal)
The results of this analysis suggest that ustekinumab is the most effective intervention, with the highest mean probability of PASI response (PASI 75: 73%, 95% CrI 67% to 79%), followed by adalimumab (PASI 75: 63%, 95% CrI 55% to 70%), etanercept (PASI 75: 40%, 95% CrI 34% to 47%) and methotrexate (PASI 75: 34%, 95% CrI 25% to 42%). Ustekinumab was statistically significantly more effective than any other agent based on relative effect estimates for PASI 75 (vs. etanercept: RR 1.78, 95% CrI 1.50 to 2.12; vs. adalimumab: RR 1.15, 95% CrI 1.01 to 1.35) and adalimumab was statistically significantly more effective than etanercept (RR 1.54, 95% CrI 1.25 to 1.88). The estimated pooled placebo absolute effect is in line with that observed, on average, across all studies in all populations.
These unadjusted results, however, do not consider an explicit adjustment for differences in placebo response rates across trials or differences across the populations (i.e. children and young people compared with adults). In the following sections, the results from the adjusted analyses are presented.
Adjustment for differences in placebo response rates across the trials
The NMA in the full population compares treatment outcomes across a large number of separate clinical trials. The reliability of these comparisons depends on the cross-trial similarity of the patient populations included in the network. An important difference between the included trials is the observed PASI response rates in the placebo arms of the trials, which is a common reference treatment across the majority of the trials. Table 41 showed that the PASI response rates in the placebo arms of the trials ranged from 0%136 to 20%.108 All of the trials varied by design, eligibility criteria, previous medication use, average age and other characteristics. All of these variations could contribute to differences in placebo response rates and, therefore, to differences in the relative efficacy of the intervention compared with placebo. However, there is no systematic way to identify the reasons for these differences. A ‘placebo creep’ phenomenon has been discussed in the literature, which identifies a relationship between placebo response rates and time since publication of the trial results. However, such a phenomenon has not been identified in the trials considered in the NMA (Figure 6). The average PASI 75 response rate in the placebo arm across all trials is 6.2%, whereas the average rate in studies of adult populations is 5.9% and in studies of children and young people is 11.1%. Three adult studies106,108,132 have substantially higher placebo response rates (approximately 18–20%) than the other studies. Four studies, including the two trials in children and young people72 and two in adults,114,116 have approximately double the average placebo rate.

FIGURE 6
Probability of a PASI 75 response in the placebo arms of trials in the NMA by publication year.
It is not clear exactly how these varying placebo rates affect treatment effects; however, it is clear that any differences will affect the relative efficacy of the interventions compared with placebo. Therefore, a potential relationship between baseline risk and relative treatment effect was explored103 in analysis 2a.
Tables 47 and 48 present the results of the model that adjusts for differences in placebo response rates. As for the unadjusted analysis (i.e. analysis 2), the baseline adjusted random-effects model was found to fit the data considerably better than the fixed-effect counterpart (DIC: 1303.7 fixed effects vs. 1177.6 random effects; total residual deviance: 473.5 fixed effects vs. 380.9 random effects). Furthermore, the 95% CRIs for the estimated mean baseline effect derived in the baseline-adjusted model do not include zero (–0.93, 95% CrI –0.97 to –0.88). This suggests that adjusting for baseline risk heterogeneity is important to explain existing between-study variation.
TABLE 47
Network meta-analysis results for analysis 2a: probability of achieving PASI 50, 75 and 90 response rates
TABLE 48
Relative effect estimates (as RRs, means and 95% CrIs) for each treatment combination for PASI 75 in the direct trial comparisons (upper diagonal) and from the NMA results for analysis 2a (lower diagonal)
The results of analysis 2a suggest that ustekinumab is the most effective intervention, with the highest mean probability of PASI response (PASI 75: 73%, 95% CrI 66% to 79%), followed by adalimumab (PASI 75: 66%, 95% CrI 58% to 74%), and etanercept (PASI 75: 41%, 95% CrI 35% to 49%) and methotrexate (PASI 75: 34%, 95% CrI 25% to 44%). Ustekinumab is statistically significantly more effective than etanercept based on relative effect estimates for PASI 75 (RR 1.77, 95% CrI 1.48 to 2.11), but not statistically significantly more effective than adalimumab (RR 1.10, 95% CrI 0.96 to 1.28). Adalimumab is also statistically significantly more effective than etanercept (RR 1.60, 95% CrI 1.31 to 1.95).
Adjusting for differences in population and placebo response rates
Although evidence from trials in both children and young people and adults contributed to the full network of evidence (effectively assuming independence between age and treatment effectiveness), it is important to recognise that the age of the population could contribute to differences in treatment efficacy. Therefore, in analysis 2b we adjusted for differences in the population and differences in placebo response rates (as the placebo response rates were considerably different in the trials of children and young people and the trials of adults). Table 49 summarises the results of this analysis in terms of PASI response outcomes for both populations. Table 50 presents the corresponding RRs for PASI 75 for children and young people.
TABLE 49
Network meta-analysis results for analysis 2b: probability of achieving PASI 50, 75 and 90 response rates in children and young people and adults
The model from analysis 2b fits the data as well as model 2a, as both present similar average total residual deviance [380.8 (2b) vs. 381.7 (2a)]. However, the DIC is substantially higher for model 2b. This suggests that this model is being penalised because of issues of parsimony. The children and young people subgroup effect is estimated not to be statistically significantly different from the adult subgroup effect, implying that the PASI absolute effect distributions of these populations overlap. This is not unexpected because of the limited number of existing studies in the population of children and young people.
The adjustment for population resulted in similar treatment rankings for children and young people when compared with the whole population results (see Table 47). The pooled placebo response rate for children and young people was estimated to be higher than that for adults (PASI 75: 12%, 95% CrI 5% to 20% in children and young people vs. 5%, 95% CrI 4% to 6% in adults), reflecting the higher placebo response rates observed in the trials in children and young people. This affects the efficacy of treatments by substantially increasing the estimated absolute PASI response rates across all treatments, but affecting the relative effects to a smaller extent. On average, PASI 75 response rates were estimated to be 10–15% higher in children and young people than in adults. The treatment rankings, however, remained unchanged. This is consistent with clinical opinion, with efficacy rates expected to be generally higher in children and young people than in adults as the biological interventions tend to work better in individuals with a lower body weight. Also, children and young people tend to have fewer comorbidities and generally have a greater exposure to ultraviolet (UV) light from participating in outside activities. The CrIs for PASI 75 response for children and young people and adults overlap, as shown in Figure 7.
The results of analysis 2b in children and young people suggest that ustekinumab is the most effective intervention with the highest mean probability of PASI response (PASI 75: 82%, 95% CrI 71% to 90%), followed by adalimumab (PASI 75: 79%, 95% CrI 64% to 90%), etanercept (PASI 75: 54%, 95% CrI 39% to 69%) and methotrexate (PASI 75: 49%, 95% CrI 31% to 68%). The relative efficacy of ustekinumab and adalimumab is similar based on relative effectiveness estimates for PASI 75 response (ADA vs. UST 45: RR 0.96, 95% CrI 0.85 to 1.05). In children and young people, ustekinumab (RR 1.54, 95% CrI 1.28 to 1.92) and adalimumab (RR 1.47, 95% CrI 1.23 to 1.79) are statistically significantly more effective than etanercept.
A consistency assessment was undertaken that involved excluding the trials of children and young people from the evidence network. This assessment indicated that the results were consistent across populations (see Appendix 7 for further details).
Summary of the findings on relative efficacy from the network meta-analysis
There was no direct trial evidence that could be used to establish the relative effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. Furthermore, there was no common comparator across the three included trials, which precluded establishing an indirect comparison between all of the interventions without drawing on evidence from other sources, namely from a different age population (i.e. adults).
Several NMA analyses were conducted to overcome the challenges involved in formally assessing the relative efficacy of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people.
First, statistical testing was performed on age subgroup efficacy data from the clinical trials in children and young people to establish whether or not it is reasonable to assume that the PASI response rates for the treatments are independent of age within the full population of children and young people, as the trials included participants of different age ranges. An indirect treatment comparison based solely on children and young people trial data for etanercept and ustekinumab was then performed and the results presented. However, this analysis was of limited use for the economic analysis as the network did not incorporate the full set of relevant interventions. Finally, a framework of analysis using different levels of evidence from the adult population was developed to address the issue of having a disconnected network structure. Previously appraised adult trial evidence was reviewed and extracted, and was assumed exchangeable with evidence from children and young adults, for inclusion in the evidence base. Two main approaches were considered, one in which the network of trials in children and young people was connected by bringing the minimum amount of evidence required from the adult population to link the three existing trials and the other in which all relevant efficacy evidence identified in adults was incorporated in the network. For each NMA model fixed- and random-effects model approaches were investigated. The latter approach was shown to be preferable, highlighting that it was important to account for variability across trials. The rate of placebo response was identified as a source of heterogeneity. Also, population-adjusted models allowed subpopulation-specific estimates to be obtained for (1) children and young people and (2) adults. The different model adjustments were explored and the age- and placebo-adjusted model was identified as the best-fitting model. For comparison and comprehensiveness, unadjusted and adjusted model results were presented.
The PASI response results were generally consistent across the different models, both adjusted and unadjusted. Overall, PASI responses were estimated to be higher for ustekinumab, followed by adalimumab and etanercept. However, there was no statistically significant difference (at the 5% significance level) between adalimumab and ustekinumab across the majority of models for PASI 75 response. Methotrexate was the least efficacious active agent, followed by placebo. The economic model in Chapter 6 uses the results for the children and young people subgroup of the placebo and population random-effects adjusted NMA (2b; see Table 49) to inform the effectiveness estimates. This NMA model was considered to provide the most appropriate set of efficacy estimates to inform the economic analysis because (1) it considers all relevant evidence, (2) it adjusts for placebo heterogeneity, (3) it adjusts for age effects and (4) it enables the estimation of age subgroup-specific effects. Scenario analyses were also conducted in which the results from the unadjusted baseline constrained model with minimum adult evidence (1b; see Table 43) are applied in the model. Partial comparisons with direct trial data and the indirect comparison reported in Indirect treatment comparison were also incorporated in a scenario analysis for completeness.
- Evidence synthesis to inform the relative efficacy of the interventions - Adalim...Evidence synthesis to inform the relative efficacy of the interventions - Adalimumab, etanercept and ustekinumab for treating plaque psoriasis in children and young people: systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...