NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
1. Acceptability and effectiveness of named interventions to increase routine vaccine uptake
1.1. Review question
1.1.1. What are the barriers to, and facilitators for, increasing the uptake of routine vaccines?
Sub-question: What is the acceptability and effectiveness of specific interventions to increase routine vaccine uptake?’
1.1.1. Introduction
The UK has a routine vaccination schedule covering key vaccinations for different stages in life including childhood, adolescence, pregnancy, and old age (65 years and older). Current practice is for healthcare practitioners to advise people to accept these vaccinations at the relevant times unless contraindicated. However, the incorrect linking of the MMR vaccine to autism resulted in a reduction in MMR vaccination which is now being reflected in an increase in the number of cases of measles. There were 991 confirmed cases of measles in England in 2018 compared with 284 in 2017 and the World Health Organization no longer considers measles 'eliminated' in the UK. Although vaccination levels in general in the UK are relatively high, levels of uptake vary between vaccines and the age groups they are targeted at. For example, 5-in-1 coverage of children measured at 5 years was 95.2% in 2019/2020, while 83.9% of Year 9 females completed the 2-dose HPV vaccination course in 2018/19. By contrast, from April 2018 to March 2019, shingles vaccine uptake for the 70-year-old routine cohort was only 31.9%, pneumococcal vaccine uptake for all people aged 65 years and over was 69.2%, and pertussis vaccine coverage in pregnant women was 68.8%. However, vaccination rates need to be actively maintained and ideally increased in the face of increasing vaccine scepticism and misinformation. The COVID-19 pandemic has also reduced routine vaccination rates and is likely to continue to disrupt routine vaccinations in the foreseeable future. In addition, certain population groups (such as some Gypsy, Roma and Travellers and migrants) have lower levels of vaccination than the general public and additional or different actions may be required to increase their vaccination rates.
Reasons for low uptake may include poor access to healthcare services; inaccurate claims about safety and effectiveness, which can lead to increased concerns and a reduction in the perceived necessity of vaccines; and insufficient capacity within the healthcare system for providing vaccinations. In addition, problems with the recording of vaccination status and poor identification of people who are eligible to be vaccinated may have contributed to this problem. While some barriers to vaccine uptake are obvious, others remain unclear and there are likely to be additional barriers that affect specific population groups, such as Gypsy, Roma and Travellers and migrants. In addition, less is known about the facilitators for vaccine uptake. Information about facilitators and the acceptability of interventions are needed to support the successful implementation of these interventions to increase uptake. This review is part of a larger review of barriers to and facilitators for vaccine uptake. It aims to examine the acceptability, implementation and effectiveness of specific named interventions as part of a mixed-methods analysis. The protocol for this review, together with the barriers and facilitators review, is detailed in Appendix A and summarised in Table 1.
1.1.2. Summary of the protocol
Table 1
SPIDER table.
1.1.3. Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Declarations of interest were recorded according to NICE’s conflicts of interest policy.
Methods specific to this review question are described in the review protocol in Appendix A and the methods document.
Please note that the review protocol also includes a quantitative question about interventions to increase uptake. This part of the work is presented in evidence review C to ensure the size of the evidence reviews remains manageable. This review is part of a larger qualitative review looking at the barriers to and facilitators for vaccine uptake.
The following additional methods apply to both qualitative reviews:
- This review refers to the UK routine vaccination schedule. The November 2019 schedule was used for these reviews and is available with the current version of the complete routine immunisation schedule.
- In this guideline, the term pregnant woman is used to include women who are pregnant as well as transgender or non-binary people who are pregnant. This terminology is used to maintain consistency with NHS websites.
- A date limit of 1990 was used for all reviews because the vaccination schedule for babies changed in 1990. This will include papers published after the MMR scandal of 1998 when attitudes to vaccinations changed in the UK and the numbers of vaccine related studies increased greatly.
- The countries of interest were limited to those in the Organisation for Economic Cooperation and Development (OECD) because less economically developed countries are likely to have different reasons for low levels of vaccine uptake associated with less well-developed healthcare systems. As a result, interventions to improve uptake and the view about barriers and facilitators to vaccine uptake in these countries are less likely to be relevant for the UK.
- They agreed that UK studies could be prioritised if a large number of studies were identified. Where there was insufficient evidence from the UK alone this prioritisation was extended to include studies based in Australia, Canada, Ireland, the Netherlands and Scandinavia (Denmark, Norway, and Sweden) because they also have universal healthcare and similar populations to the UK.
- To make analysis easier the review work was divided into categories based on subgroups listed in the protocol. These were: pregnant women; people aged 65 years and older; 0–5 year olds and 11–8 year olds.
- The decision to only look at UK evidence or the extended OECD subset was made at the subgroup level so, for example, where we found sufficient evidence for the views of parents concerning HPV vaccinations we didn’t look for papers on this topic in the wider literature. The decision that there is sufficient evidence was made based on the number and richness of the included studies in consultation with the committee to ensure that they are able to make recommendations.
- The committee noted that it was the presence of a vaccination against a disease on the routine schedule rather than the formulation of the vaccination that was important and therefore studies would not be excluded for using different formulations to the UK.
- Routine vaccination schedules of countries other than the UK were checked using the WHO vaccine-preventable diseases: monitoring system unless a more up -to-date, approved, national/regional immunisation schedule is identified online. The routine vaccination schedule covers all routine vaccines from 8 weeks to 70 years old and includes the pertussis vaccine for pregnant women. People who are also eligible for selective immunisation programmes (e.g., high-risk groups) or additional vaccines will be included for routine vaccines only.
- The committee agreed that studies from the OECD would be judged as highly relevant initially and then downgraded at the study level if there was a reason to believe that the individual study was not completely relevant to the UK population. In addition, a finding identified from an otherwise highly relevant or relevant study could be downgraded if it was not relevant to the UK population. Committee input was used to determine where it was appropriate to downgrade in this manner.
- Where a study was conducted in a country which has some differences in routine vaccine schedule compared to the UK but reports on barriers and facilitators to vaccine uptake in general, rather than a specific vaccine, it was included in the review and not downgraded.
- For studies looking at specific vaccines to be considered for inclusion, the vaccinations included in the study must be in the routine vaccination schedule of the UK and the country where the study was conducted.
- Finding from open ended questions from questionnaires were only included in the qualitative reviews when insufficient evidence is available from studies using focus groups and interviews because these usually provide a much richer source of data than open-ended questions in surveys.
- The committee agreed not to include grey literature in the search for this topic because they thought it would be time consuming to identify and that it would be hard to find relevant literature. They agreed that if insufficient evidence is identified from the included study types, they would consider a focused call for evidence instead or look at indirect evidence.
- The committee agreed that the barriers may be perceived or actual barriers (e.g., the individual may think that access is a problem because clinics aren’t’ available at convenient times/locations but if this is not the case in their area the barrier is one of perception rather than an actual physical barrier).
- Catch up campaigns included in this guideline are as follows: opportunistic campaigns for people who missed a vaccination, and catch-up campaigns in under-vaccinated groups.
- The scope of this guideline does not include flu vaccination as that is covered by another guideline (NICE flu guideline NG103).
The following additional methods apply to this qualitative review specifically:
- Qualitative studies were included in this review if they examined a specific intervention programme and had an accompanying quantitative study which reported on vaccine uptake outcomes (see protocol deviation below).
- Qualitative studies from the USA were excluded as the committee thought that that views about vaccines and vaccinations may differ due to differences between the healthcare systems in the USA and the UK. As such, the results from USA-based studies may not be generalisable to the UK population.
- Quantitative data from papers associated with the interventions identified in the qualitative part of this review are included, and quality assessed using GRADE where appropriate.
- Data for the Celebrate and Protect reminders programme for children aged 0–5 years was only available as part of a non-peer-reviewed report (Gibson 2014) and outcomes were therefore considered at higher risk of bias. This report stated the % vaccine uptake, but not the number of children in each arm. The percentages have therefore been reported to give the committee an indication of the effectiveness of the intervention, and outcomes were quality assessed using a modified version of GRADE. Quality was therefore based on risk of bias, directness and heterogeneity but not imprecision as it was not possible to calculate risk ratios.
- A mixed methods approach was taken to assess the effectiveness of an intervention alongside views of different aspects of that intervention. Diagrams were created which outlined the main aspects of each intervention. These were linked to the main themes extracted from the qualitative studies and examples were included to highlight people’s views in relation to those themes. Examples were coded to demonstrate which groups of people had reported those views (such as parents, healthcare staff and young people). For the interventions for 11–18 year olds, forest plots were included in the diagram to show whether the intervention was effective. For the 0–5 years group (Celebrate and Protect programme), insufficient data was available to produce a forest plot and so the differences in percentage vaccine uptake per arm was presented below the diagram.
- Two studies (Chantler 2020 and Gibson 2014) were cluster non-randomised trials. For these studies, a modified risk of bias assessment was used. This checklist was composed of the ROBINS-I tool for non-randomised trials, with an additional section related to clustering methods taken from the Cochrane Cluster Risk of Bias 2.0 tool.
- Where cluster RCTs were included any data provided that was already adjusted for clustering was presented. Otherwise we adjusted for clustering using an ICCs supplied in the studies or 0.05 otherwise because this was the most common ICC in the education review (evidence review E) and the MMR decision aid paper (Shourie 2013) was already included in that review with this adjustment.
Protocol deviation
The quantitative paper reporting on the effectiveness of incentivised consent forms (Forster 2017) reported consent form return as an outcome but did not report vaccine uptake. Although consent form return was not stated as an outcome in the quantitative protocol, this was included in the current review to provide an indication of the effectiveness of the intervention in the absence of specific information about vaccine uptake. The quality of this outcome was subsequently downgraded to relevant due to being partially indirectly applicable to the review.
1.1.4. Qualitative and quantitative evidence
A literature search was conducted which identified 9226 articles. Of these, 505 potentially relevant qualitative studies were identified after screening the titles and abstracts against the review protocol. Once assessed in full 5 qualitative studies matched the protocol for this part of the review because they accompanied a specific intervention to increase uptake. (See evidence review B for included studies for the barriers and facilitators component of this review question.)
One qualitative study examined the views of participants of a reminder programme for the vaccination of babies and children aged 0–5 years, and another assessed the use of a parental decision aid for the MMR vaccine. Three papers evaluated HPV vaccination programmes for young people aged 11–18 years, 1 comparing the use of e-consent forms to standard paper consent forms and 2 papers for the same intervention which offered a financial incentive for young people who returned their consent forms.
The searches were re-run in April 2021 and 3 additional papers were identified as part of the reruns, all of which evaluated the use of the same HPV vaccination programme with a new method of gaining consent where all young people were invited to vaccination sessions, irrespective of whether they had returned a signed consent form. No evidence was found for pregnant women or people aged 65 years and older. All included evidence was based in the UK.
Quantitative evidence was available for each of the interventions identified in the qualitative evidence search. Further information about these quantitative studies is included in the section below.
See Appendix C for a diagram of the qualitative evidence study selection.
1.1.4.1. Included studies
Please refer to the complete routine immunisation schedule for an explanation of the abbreviations of vaccine names used below.
Babies and children aged 0–5 years old
Two qualitative studies were included, each with an associated quantitative paper. One study evaluated the use of the Celebrate and Protect vaccine reminders programme for childhood vaccines, and the other examined the use of a web-based decision aid aimed at parents whose children were eligible for the MMR vaccine.
The Celebrate and Protect programme
The programme involved reminders being sent to the parents or carers of a child before their scheduled vaccinations. The first reminder was sent in the form of a celebration card for newborn babies with a reminder to contact their GP and book a 6–8 week check. Birthday cards were sent to 1 year old children with a reminder to book vaccinations and to 4 year olds who had not yet received their immunisations. Celebration/reminder cards also contained information signposting parents and carers to the Personal Child Health Record and immunisation websites.
The qualitative study used semi-structured interviews with policymakers and practitioners and focus groups with parents and carers. The quantitative study reported on vaccine uptake at 12 months, 24 months and 5 years of age. The quantitative study was a non-peer reviewed article and so the quality of vaccine uptake outcomes were downgraded for risk of bias.
The web-based decision aid
Parents were sent a flyer that provided them with a website address and password so they could access the decision aid. The decision aid provided parents with background information on measles, mumps and rubella as well as the immunisation schedule and how the MMR vaccine works. Information was also provided on common symptoms and complications of each of the three diseases, as well as safety and side-effects of the vaccine. Interactive content was also included to help with the decision- making process, prompting parents to consider their reasons for or against vaccination and to record their intentions towards the MMR vaccine.
The qualitative study used a combination of questionnaires and semi-structured telephone interviews with parents to examine their views on the content of the intervention and its effect on decision making. Outcomes were assessed 1 week after parents accessed the decision aid.
See Table 2 and Table 3 for a summary of the characteristics of these studies.
Young people aged 11–18 years old
Three qualitative studies were included. One mixed-methods study evaluated an e-consent school-based intervention for HPV vaccinations. Two qualitative studies examined the same school-based HPV intervention, which promoted financial incentives for consent form return.
The e-consent intervention
The intervention provided parents and carers with a link to an online portal which included an electronic consent form and information about the vaccination programme. Parents and carers could use the portal to register their child and either agree to, or decline, the vaccination. The portal also gave nurses the ability to screen consent form return and to update records during immunisation sessions.
For the qualitative outcomes in the mixed methods study for the e-consent intervention, semi-structured interviews were held with Trust staff, parents or carers and young people. Focus groups were also conducted with some of the young people. The quantitative outcome of the study was number of people vaccinated at school immunisation sessions.
The financial incentives intervention
Girls in year 8 of secondary school (aged 12–13 years) were given standard information about the HPV vaccine and a consent form to be signed by their parents or carers. Girls were told (verbally by their teachers and via a letter provided with the consent form) that if they returned the signed consent form they would be entered into a prize draw to win one of several £50 shopping vouchers. Girls were entered into the draw regardless of whether the consent form said ‘yes’ or ‘no’ to the vaccination.
The first qualitative study, during the pilot phase of the project (Rockliffe 2018), gave questionnaires to girls and their parents to explore their opinions on the intervention. Staff members involved in running the intervention took part in telephone interviews. The second study (Rockliffe 2020), completed when the intervention was repeated the following year, used focus groups and questionnaires to explore girls’ perceptions of the incentives. One quantitative paper reported on the effectiveness of this intervention. The outcome from this paper was consent form return, rather than vaccine uptake, but was included to provide an indication of effectiveness in the absence of any information on uptake.
The intervention with a new method of obtaining consent
All female students in year 8 of secondary school (aged 12–13 years) were given an information leaflet about the HPV vaccination programme alongside a consent form for their parents or carers to either consent or refuse to vaccination. Vaccination sessions were modified so that all young people were invited to the vaccination session, rather than just those who had returned a consent form. During the vaccination session, young people with a consent form signed by their parent received the vaccine. Those who wanted to receive the vaccine but had not returned a parental consent form spent more time with an immunisation nurse who attempted to contact a parent or carer by telephone to ask for verbal parental consent. If verbal consent was obtained then the young person was given the vaccine. If a parent or carer could not be contacted, then young people were assessed for competence by the immunisation nurse. This involved a discussion of their understanding of the purpose of the vaccine and possible side effects, and any health issues that needed to be taken into consideration. If a young person was assessed as competent, reported that they had discussed the vaccine with their parents or carers, and said that it would not cause disagreement within the family, they could provide written consent and be given the vaccine. Young people who were not deemed competent, or indicated that vaccination would cause disagreement at home, were not vaccinated and instead were given information about community-based clinics run by the immunisation nurses where the vaccine could be administered.
The 3 qualitative studies used semi-structured interviews with immunisation team members, school staff, parents and young people to examine people’s views of the intervention and understanding of the self-consent procedure. The quantitative study reported vaccine uptake in the 2 areas that took part in the intervention using an uncontrolled-before-after design which did not include a comparator arm.
See Table 4, and Table 5 for a summary of the characteristics of these included studies.
The references for included studies are listed in Section 1.1.14
1.1.4.2. Excluded studies
The reasons for excluding studies at the full text stage are detailed in appendix J. Common reasons for excluding studies were ineligible study designs and participants with age ranges that did not overlap age ranges within the routine immunisation schedule.
1.1.5. Summary of studies included in the qualitative and quantitative reviews
Babies and children aged 0–5 years
Table 2
Summary of characteristics of included qualitative studies for vaccination of babies and children aged 0–5 years old.
Table 3
Summary of characteristics of included quantitative studies for vaccination of babies and children aged 0–5 years old.
Young people aged 11–18 years
Table 4
Summary of characteristics of included qualitative studies for vaccination of young people aged 11–18 years old.
Table 5
Summary of characteristics of included quantitative studies for vaccination of young people aged 11–18 years old.
See Appendix D for more details about the included studies.
1.1.6. Summary of the qualitative evidence
Babies and children aged 0–5 years

Figure 1
Mixed methods summary of the effectiveness of the Celebrate and Protect reminders programme on childhood vaccine uptake, and the views of people involved in trialling the intervention. See the findings in Table 6 for more details.
| Vaccine | Effect | Vaccine | Effect | Vaccine | Effect |
|---|---|---|---|---|---|
| 12 months of age | 24 months of age | 5 years of age | |||
| Diphtheria, tetanus, pertussis, polio, Hib | Uptake 2.3 percentage points higher for control | Hib and Meningitis C | Uptake 1.1 percentage points higher for Celebrate & Protect | MMR 1st dose | Uptake 3.6 percentage points higher for Celebrate & Protect |
| Meningitis C | Uptake 2.6 percentage points higher for control | MMR 1st dose | Uptake 1.8 percentage points higher for Celebrate & Protect | MMR 2nd dose | Uptake 1.8 percentage points higher for Celebrate & Protect |
Table 6
Summary of the themes for vaccination of children aged 0–5 years using the Celebrate and Protect (reminder) programme.
See Appendix F for full GRADE-CERQual tables

Figure 2
Mixed methods summary of the effectiveness of an MMR web-based decision aid on MMR vaccine uptake, and the views of people involved in trialling the intervention. See the findings in Table 7 for more details.
Table 7
Summary of the themes for vaccination of children aged 0–5 years using an MMR web-based decision aid.
See Appendix F for full GRADE-CERQual tables
Young people aged 11–18 years
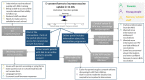
Figure 3
Mixed methods summary of the effectiveness of e-consent forms on HPV vaccine uptake, and the views of people involved in trialling the intervention. See the findings in Table 8 for more details.
Table 8
Summary of the themes for vaccinating young people aged 11–18 years using e-consent forms.
See 110Appendix F for full GRADE-CERQual tables

Figure 4
Mixed methods summary of the effectiveness of incentivised consent forms on HPV vaccine uptake, and the views of people involved in trialling the intervention. See the findings in Table 9 for more details.
Table 9
Summary of the themes for vaccinations of for young people aged 11–18 years using incentivised consent forms.
See Appendix F for full GRADE-CERQual tables.
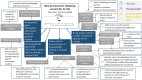
Figure 5
Mixed methods summary of the effectiveness of using a new process to obtain consent on vaccination day. See the findings in Table 9 for more details.
Table 10
Summary of the themes for vaccinations for young people aged 11–18 using a new process to obtain consent on vaccination day.
See Appendix F for full GRADE-CERQual tables.
1.1.7. Summary of the quantitative evidence
Babies and children aged 0–5 years
Table 11
Babies and children aged 0–5 years using reminders (Celebrate and Protect Programme).
See Appendix F for full GRADE tables.
Table 12
Babies and children aged 0–5 years using an MMR web-based decision aid.
See Appendix F for full GRADE tables
Young people aged 11–18 years
Table 13
Young people aged 11–18 years using electronic consent forms.
See Appendix F for full GRADE tables
Table 14
Young people aged 11–18 years using incentivised consent forms.
See Appendix F for full GRADE tables
Table 15
Young people aged 11–18 years using a new process to obtain consent on vaccination day.
See Appendix F for full GRADE tables
1.1.8. Economic evidence
A single systematic review was conducted to identify economic evaluations relevant to any of the quantitative review questions in the guideline. The search returned 5,716 records which were sifted against the review protocol. Of these publications 5,669 were excluded based on title and abstract. On full paper inspection 43 studies did not meet the inclusion criteria for any review question. Inclusion was restricted to cost-utility analyses from OECD countries comparing interventions to increase vaccine uptake for vaccines in the UK immunisation schedule as described in the green book. Four published economic analyses were included in the evidence synthesis.
Due to a lack of cost-utility evidence in children, an additional inclusion set was used to identify studies in children and adolescents (0–18 years), where outcomes were not restricted to QALYs only (and therefore cost-effectiveness studies were also included). An additional six studies from the search were included on this basis to provide evidence in the younger population.
The search was rerun in April 2021 to identify any newly published papers and returned 544 publications, of which 541 were excluded based on title and abstract and two were excluded at the full text inspection. One additional published cost-utility analysis from this search was included in the evidence synthesis.
1.1.8.1. Included studies
None of the 11 studies identified in the systematic review were relevant to this review question.
1.1.8.2. Excluded studies
A list of studies excluded at full text from the cost-effectiveness review can be found in Appendix J.
1.1.9. Economic model
The committee discussed incentives for consent form return for school-based vaccinations and, due to the anticipated resource impact, a costing analysis was undertaken to better estimate the costs associated with this intervention.
The incremental cost per additional person vaccinated against was calculated for incentives plus phone reminders compared with phone reminders only. Positive consent form return was used as a proxy for vaccination. Effectiveness evidence for the incentive intervention was taken from the Forster 2017 study where the incentive was a 1 in 10 chance of winning a £50 voucher. Alternative incentive values were also considered in the costing analysis, however there are limitations to these results as the effectiveness data is only available for the base-case analysis.
The cost per additional person vaccinated for each incentive value scenario and under two baseline uptake scenarios is presented in Table 16. Further details of the costing analysis are provided in Appendix I.
Table 16
Incremental cost per additional person vaccinated.
1.1.10. Economic evidence statements
One original costing analysis found that for school-aged children eligible for the HPV vaccination, incentivising consent form return resulted in lower costs and higher vaccine uptake if the incentive was either a free-to-provide incentive or a 1 per school chance at winning a £50 voucher. For other incentive scenarios (a 1 in 10 chance of winning a £50 voucher, or a fixed amount of £3 per student) the cost per additional person vaccinated was between £13.07 and £97.81.
1.1.11. The committee's discussion and interpretation of the evidence
1.1.11.1. The outcomes that matter most
For the qualitative section of the review, views of young people, parents and carers and healthcare practitioners were all considered. For the HPV vaccine, the views of both young people and their parents and carers were considered particularly important because they are the groups responsible for consenting to the vaccine and for ensuring that consent forms are returned. For babies and children aged 0–5 years the views of the parents and carers were considered most important. However, the views of staff involved in delivering the interventions were also important, especially where they covered factors relating to implementation. The committee agreed that the most important findings were those concerning the acceptability of an intervention to its target; issues to do with implementing the intervention; and whether there were unintended consequences.
For the quantitative studies, the primary outcome was vaccine uptake. The committee agreed that this outcome was the most important for individuals, their parents and carers (as appropriate), and healthcare practitioners because the aim of this guideline is to increase vaccine uptake. None of the included studies reported the protocol’s secondary outcomes, which were the proportion of people offered vaccinations and the numbers of people who develop the diseases the vaccines are aimed at preventing. Offers of vaccination was not considered as important as uptake because an offer may not necessarily result in a vaccination. Consent form return, and proportion of consent forms returned that agreed to vaccination, were not included in the protocol, but in the absence of information on vaccine uptake, were also considered important by the committee (see protocol deviation).
1.1.11.2. The quality of the evidence
Evidence was only identified for interventions targeted at young people aged 11–18 years and for babies and children aged 0–5 years. No qualitative papers accompanying interventions were identified for people aged 65 years and over or for pregnant women.
Babies and children aged 0–5 years
The qualitative evidence for the Celebrate and Protect reminders programme was low to very low quality and was from a single study which provided limited information about recruitment methods. The quantitative evidence for vaccine uptake was very low quality, as it was from a non-peer-reviewed report which did not report how many participants were in the control arm or how they were recruited. The limited details on the methods used for recruitment meant that it was not possible to calculate effect sizes and confidence intervals for vaccine uptake, and so the only data that was available was percentage uptake per trial arm. Percentage vaccine uptake was not one of the outcomes stated in the protocol, but the study was included to provide the committee with an indication of the effectiveness of the intervention, with the quality of the outcomes downgraded for risk of bias. This approach was taken with the agreement of the committee.
Qualitative evidence for the use of an MMR web-based decision aid was very low quality and the evidence for vaccine uptake was low quality. Very few parents were included in the semi-structured interviews used in the study, and only two themes were identified with limited evidence to support each theme. The qualitative evidence was from a pilot of the intervention which did not provide information on vaccine uptake, and so the quantitative evidence was instead provided from a study published 3 years after the feasibility study had been completed. The effectiveness data is therefore from a different group to those who provided their views on the intervention; however, the newer study does not report changing the intervention from the format used in the pilot. Finally, this intervention was carried out on a population with high vaccine uptake, with 100% of children being given the vaccine in the intervention arm, and 98% in the control arm. It was therefore difficult to determine the effectiveness of the decision aid, which could potentially show a greater effect in a population with lower vaccine uptake.
Young people aged 11–18 years
For young people aged 11–18, the qualitative evidence for the use of e-consent forms was low to very low quality and the quantitative evidence for vaccine uptake was very low quality. The evidence was from a mixed-methods study of a pilot project designed to evaluate the implementation of the intervention. The quality of qualitative outcomes was downgraded as they were obtained from a single study with limited detail provided to support the themes. While the views on effectiveness of the intervention were directly applicable to the review, the views on implementation were only relevant (rather than highly relevant) as the part of the intervention which allowed nurses to monitor consent form return and update vaccination records was not fully functional during the pilot phase.
Qualitative evidence for the use of incentivised consent forms for the HPV vaccine ranged from very low to moderate quality, and the quantitative outcome was low quality. The effectiveness of this intervention was assessed by the number of consent forms returned, rather than vaccine uptake. Although this is not the stated outcome for this review, the committee discussed whether consent form return was an appropriate outcome that would provide an accurate reflection of changes in vaccine uptake. It was decided that, although vaccine consent forms could be returned with non-consent to the vaccine, it was likely that many of those returned would consent to vaccination. This was supported by an additional outcome in the incentivised consent form study, which reported the proportion of consent forms returned with a positive outcome (consented to vaccination). In the incentivised consent form arm, not only were more consent forms returned, but there was a higher proportion of positive consent forms returned in the intervention arm than in the control arm. In addition, in both arms over 85% of the returned forms gave consent for vaccination. Consent form return was therefore included as a proxy outcome for vaccine uptake, with the quality of the evidence downgraded once for indirectness. It was also highlighted that the evidence for this intervention was from an area of relatively low vaccine uptake. Although there was no evidence for the use of incentives in areas of higher uptake, the committee decided that, given the effectiveness of the intervention, incentives should be recommended to providers in all areas (see below for more details).
As there was limited evidence on this subject, the committee also decided to include a research recommendation to examine the effectiveness and acceptability of school-based incentive schemes. They included both financial and non -financial incentives and specified that the incentives did not have to be aimed at incentivising uptake directly but could be aimed at promoting other behaviours (for example, consent form return) that ultimately result in increased vaccine uptake. (See below for the committee’s discussion about the potential issues raised by the use of financial incentives for vaccine uptake.) The committee discussed whether lower levels of incentive would have been equally effective in Forster 2017; and wondered at what level would the incentive cease to be effective. As a result, the economist carried out a costing exercise to estimate the resource implications of recommending incentives to increase consent form return (see the cost-effectiveness and resource use section below). This involved a range of scenarios and the remaining uncertainty around the level of incentive needed for cost-effectiveness resulted in the committee adding consideration of the levels, as well as types, of incentive that could be effective to the research recommendation. This research recommendation is explained in detail in Appendix K
Qualitative evidence for the use of new methods of obtaining consent (Audrey 2020, Audrey 2021, Fisher 2020a) ranged from very low to moderate quality and the quantitative outcome was very low quality. Both qualitative and quantitative evidence was directly applicable to the review, and while the quantitative evidence did not show an increase in vaccine uptake with the new methods of obtaining consent, the committee thought that the qualitative evidence raised some important issues that are relevant to the current methods used for school-based vaccinations. The new methods of obtaining consent described in the study are similar to those used in current practice and so the committee decided that the qualitative findings were directly relevant and could be applied to the current processes involved in obtaining consent for school-based vaccinations.
People aged 65 years and over and pregnant women
As no evidence was identified for either people aged 65 years and over or pregnant women, the committee could not make recommendations on specific interventions to increase vaccination uptake in either of these groups based on this review. Limited qualitative and quantitative evidence was identified for these groups in the intervention reviews and the main qualitative review on barriers to and facilitators for vaccine uptake. The committee therefore decided to make a research recommendation for each of these populations to identify whether there are any interventions that are both acceptable and effective at increasing vaccine uptake (for the research recommendation for people aged 65 years and over, see Appendix K in the review B on the barriers to and facilitators for vaccine uptake for more details; for pregnant women see Appendix K in the vaccinations for pregnant women review F for more details).
1.1.11.3. Barriers and facilitators for routine vaccinations
Babies and children aged 0–5 years
For babies and children, the qualitative evidence associated with the Celebrate and Protect intervention indicated that contact with parents soon after the birth of a child can be a problem, and some parents highlighted that they would like more, balanced, information about vaccinations. It was unclear from the quantitative evidence whether the intervention was effective at increasing uptake as the data was reported as % with no indication of variation. The committee agreed that there was insufficient evidence of benefit (increased vaccine uptake) to support recommending this particular intervention. They also noted that the funding for this intervention came from a pharmaceutical company and that this may have affected parents’ responses to the intervention and, as a result, its effectiveness.
When discussing birthday celebration and reminder cards as a method of increasing childhood vaccination uptake, the committee highlighted concerns over a potential scenario where a child has died, and the relevant information has yet to be updated in their GPs system, resulting in a celebration card being sent to the family. Although this could also happen for vaccine reminder letters, the committee thought that receiving a celebration card for a child who has died would be a much worse scenario and would cause unnecessary pain to the bereaved family. The committee noted that they had already recommended using invitations/ reminders for vaccination with information based on the evidence in the reminders and education reviews (evidence reviews C and E respectively) and that these recommendations did not need amending based on the celebrate and protect intervention.
The quantitative evidence for the MMR decision aid had already been considered in the education review (evidence review E) alongside other studies looking at decision aids. The evidence from Shourie 2013 could not differentiate vaccine uptake between people using the MMR decision aid and usual care, but this may be due at least in part to the very high baseline vaccination rate in this study. The committee noted that the main themes identified in the qualitative evidence from this review showed that parents thought the decision aid helped them make informed decisions about vaccination and that that the presentation of information about the benefits and harms of vaccination was useful. The desire to make an informed decision based on information that is perceived to be balanced was also reflected in the main barriers and facilitators review (evidence review B). Based on the evidence in review E, the committee decided against making a specific separate recommendation in favour of using decision aids, however they agreed when they are available on trusted sites, they could potentially be a useful method of helping people make decisions about whether to be vaccinated or have their children vaccinated. This was supported by the qualitative findings with parents highlighting how the decision aid helped with decision making and reduced the need for them to ask further questions before reaching a decision.
Young people aged 11–18 years
One of the main barriers to young people receiving the HPV vaccine in schools relates to difficulties with gaining consent, as is the case when consent forms are not returned. This leads to immunisation providers spending lots of time chasing up non-responders to try to obtain consent. Evidence for the use of incentivised consent forms showed an increase in the number of forms returned when entry into a prize draw was offered. The committee therefore thought that this may be a useful method of increasing the number of forms returned. The committee discussed whether there were potential ethical issues surrounding the offer of financial incentives relating to vaccinations (see evidence review G for more discussion about this issue). However, it was highlighted that the incentive used in the evidence was based on consent form return rather than receiving the vaccination. The committee decided that this was a more acceptable form of intervention as it was promoting decision making about the vaccination, rather than the vaccination itself. However, it was noted that financial incentives may not be appropriate in all settings, such as faith schools, where a prize draw could be considered a form of gambling. The committee therefore decided not to specify what the intervention should be and instead the decision on exactly what incentive is offered can be made at a local level, by people who have an awareness of the particular school, the local community and its beliefs. The committee discussed how other potential incentives could include vouchers or lunch passes, which may be more acceptable in some settings than financial rewards. In addition, they also did not specify the target of the intervention. In the Forster studies (2018 and 2020) the intervention was aimed at students, but other interventions may be better suited to targeting parents and so the committee left this open for the providers to decide. Finally, the recommendation was not limited to areas of low uptake because the committee noted that the use of incentives for consent form uptake could also provide benefits in areas of high uptake by reducing the time needed for the immunisation team to chase up non-responders.
The committee considered whether e-consent forms could remove some of the barriers associated with non-return of paper consent forms. However, the evidence highlighted that while some parents preferred e-consent forms, others may not have access to the technology needed to access and return them online. Given that the evidence could not differentiate between vaccine uptake when e-consent or paper consent forms were used, the committee decided that they could not make a recommendation in favour of a single option. Instead, it thought that schools would be better placed to decide on the best option to suit their local community and decided against making a recommendation based on methods of consent form return, although they noted that e-consent forms are already in use in many areas.
The evidence for both e-consent and incentivised consent form return highlighted the importance of young people being made aware of vaccines and the vaccination process. One of the main themes relating to the HPV vaccine was that young people want to be involved in discussions surrounding the vaccine, such as the benefits and harms associated with vaccination. As this was a common theme in both qualitative studies and had been raised in the main barriers and facilitators review as well, the committee decided it was important to highlight this in a recommendation to try to help parents, carers and young people make an informed choice about vaccination. The committee decided that information about Gillick competence should also be included so that parents and young people are all fully aware of all the options surrounding vaccine consent. An additional recommendation was made, specifically aimed at young people, which highlighted the importance of school-based education. Although the qualitative evidence reported a range of views on the most appropriate method of giving young people information about vaccination, school-based education was commonly stated as one of these options. It was the committee’s experience that this is standard practice in some schools, and they agreed that this was an important way of providing information about vaccinations delivered in school settings to children of appropriate ages and young people. They noted that the information provided could be tailored to their needs and level of understanding as young people would be able to understand more complex information than children.
The committee agreed that consent is a particularly important issue relating to school-based vaccinations because the person giving consent may not be present at the time of the vaccination. In addition, the evidence from studies looking at vaccinations for 11–18 year olds in this review, alongside the evidence from the qualitative review (evidence review B), highlighted that while immunisation teams are well trained to assess Gillick competence, some people do not feel comfortable with the concept of young people being allowed to self-consent for vaccination. This is therefore an issue which needs to be carefully considered.
Although the aim of the recommendation for incentivised consent form return is to increase the number of young people who return their vaccination forms, there will still be times when a young person does not have a signed consent form at the time of a school vaccination session. If a nurse is unable to contact the parents or carers of the young person to ask for their views, then they can instead make an assessment over whether the young person is capable of making the choice about vaccination themselves. The studies on new methods of obtaining consent suggested that this could take place at the time of a routine vaccination session, and the committee agreed that, where possible this would be useful. However, they thought that time constraints would often make this difficult to achieve in practice. Instead, they agreed that Gillick competence assessments and vaccinations for those who are considered competent should be offered at the earliest opportunity, which could either be during a routine vaccination session or during catch-up sessions for young people who have been identified as not up to date with their vaccinations.
An alternative scenario for assessing Gillick competence is when the parent or carer has returned the consent form and has refused vaccination, but the young person has different views to their parents and wishes to be vaccinated. The committee thought that if a young person is assessed as competent then, where possible, they should still be offered vaccination and that this could be assessed as part of the catch-up process. To do otherwise might be discriminatory as the young people who lacked parental consent (or refusal) could be assessed for Gillick competence but the young people whose parents had refused vaccination would be denied this opportunity. However, the committee were aware of the potential issues associated with going against parental wishes. In addition, although the committee thought that offering a Gillick competence assessment is important, their experience was that there are not always clear systems in place to ensure that vaccination teams feel supported when carrying out this assessment. The studies on new methods of obtaining consent included processes that immunisation nurses had to follow to guide their Gillick competence assessments, such as young people being asked if they had discussed vaccination with their family and whether being vaccinated could cause conflict with their parents or carers. The immunisation nurses reported that this helped them to make decisions on a person’s capacity to consent. As a result, the committee agreed that it was important that school aged immunisation providers have a policy on Gillick competence in place and that this should include guidance about what action to take when a young person’s vaccination preference is different from their parents to help to support the decisions of the immunisation teams in the schools. They also included cross references to help ensure that the assessment of Gillick competence is be carried out in line with national guidance, such as that set out in the Green book and by the General Medical Council.
When discussing consent, the committee noted that parental refusal might be limited to specific vaccinations (for example, HPV) and that that there are some settings, such as faith schools, where it may be deemed unacceptable for young people to be vaccinated if this is against the parents’ wishes. This was considered a key issue, as assessing for Gillick competence in this scenario could potentially damage the ability of the immunisation team to access the school for other vaccination campaigns. The committee highlighted a qualitative study (Chantler 2019) which investigated methods of obtaining consent for school vaccinations. The findings of this study reinforced the concerns of some schools about young people being allowed to self-consent to vaccination, and highlighted that, in some cultures, a young person may feel unable to go against the decisions of their parents. It is therefore important that school aged immunisation providers and vaccination teams tailor their approach to Gillick competence assessments based on local populations. This will maximise the number of young people that are vaccinated but also ensure that the actions of vaccination teams do not have any unintended consequences.
1.1.11.4. Cost effectiveness and resource use
In the absence of published economic evidence for this section, the committee used their expertise to inform discussion around the expected resource and cost impact of these recommendations.
The committee considered the evidence on incentives and recommended that incentives be offered by providers as a method to increase the number of consent forms returned for school-based vaccinations. A costing analysis was conducted to estimate the resource implications of recommending incentives to increase consent form return. The costing analysis compared vaccine uptake of incentives followed by phone reminders with phone reminders alone, using positive consent form return as a proxy for vaccine uptake as agreed by the committee. Incremental cost per additional person vaccinated was calculated for different incentive values, with the base-case assumption of a 1 in 10 chance of winning a £50 voucher being associated with an ICER of £97.81. Lower value incentive scenarios selected by the committee (one £50 voucher per school or free school-based perks) were also modelled and were cost saving when compared to phone reminders only, as the increased form returns with incentives led to lower numbers of nurse phone calls required than without incentives. However, the committee noted that the efficacy evidence for incentives was based on the base-case incentive value of a 1 in 10 chance of winning a £50 voucher, and that lower value incentives may not be as effective. Nonetheless, the committee felt that whilst these lower value incentives would be unlikely to change the mind of someone who had actively decided not to be vaccinated, they would likely be effective in improving consent form returns from people with no objections to vaccination that had simply neglected to complete the necessary paperwork. This would likely save money downstream from providers having to contact less families to chase up unreturned consent forms.
A low uptake scenario was also conducted using data from a local authority with low vaccine uptake. The ICER associated with this low uptake scenario was £34.07, suggesting that the combination of incentives plus reminders is more cost-effective in populations with low vaccine uptake. The committee felt that although the intervention was more cost-effective in populations with low uptake areas, it was justified to recommend incentives for consent form return in the general population. The committee considered that one of the reasons some areas have higher uptake than others is that the school nursing teams may already be doing a lot of outreach work and phone call reminders to return consent forms, which is very resource intensive. In this situation the incentive intervention was considered to be useful as it would likely reduce the number of phone calls required and displace some of the associated costs.
The committee discussed the issue of consent for school-based vaccinations and recommended that providers ensure sufficient information, including information on who can consent for vaccination, is provided to both young people and their parents for decision making, and that parents are encouraged to discuss vaccination with their children. This recommendation is unlikely to have resource implications as this additional information can be included with the information already provided and detailed in the recommendation on what invitations should contain.
The committee recommended that providers have a policy in place to support school immunisation teams in assessing Gillick competence. This recommendation is likely to require some development from vaccination providers to communicate a consistent policy to their immunisation teams, but this is anticipated to be associated with minimal additional costs.
The committee recommended that, in the absence of parental consent to vaccination, children and young people should be offered the opportunity to be assessed for Gillick competence for the ability to self-consent. Due to time constraints in the routine vaccination sessions, the committee suggested that these opportunities are provided at catch-up vaccination sessions. School-based catch-up vaccination sessions are often offered in usual practice already and provided school nursing teams are already present at the sessions, it is not anticipated that adding this opportunity to be assessed for Gillick competence will have additional resource implications.
The committee discussed consent for vaccinations for other groups, such as those who may need additional support to consent or those who lack capacity to consent, and recommended that the NICE guidance on these groups should be followed to ensure people receive the appropriate vaccinations. Since this recommendation refers to existing NICE guidance it is not expected to have additional resource use, as the support required in the vaccination context would be the same support these people would receive for making other healthcare decisions.
1.1.11.5. Other factors the committee took into account
Catch up sessions and tailoring immunisation programmes
Catch-up sessions are part of routine practice, and the committee discussed the importance of these to ensure that children and young people are given additional opportunities to be up to date with the routine adolescent vaccination schedule. In addition, they note that catch-up sessions in schools would ensure that children and young people who are not up to date with their vaccinations have other opportunities to be vaccinated. These sessions are currently limited to children and young people who had missed school-aged vaccinations, but they could be expanded to provide opportunities to catch up on earlier preschool vaccinations. At the moment, children and young people have to be directed to their GP to be offered these vaccinations. The committee noted that some children or young people will be unable to attend the school-aged catch-up sessions, for example due to sickness, exclusion or extended leave, so alternative provisions are necessary to ensure that they can be offered their overdue vaccinations. This could involve signposting to GPs or other places where the vaccinations are available. To help with identifying these children and young people, CHIS can provide vaccination histories to providers and the committee made a recommendation for CHIS to do so.
Catchup sessions commonly occur in schools but can be carried out in other settings, such as GP surgeries. The committee noted that very little evidence for catch-up sessions had been identified as part of the evidence reviews for this guideline. One study (Altinoluk-Davis 2020) was identified as part of evidence review D (on improving access to vaccinations). It was carried out in the UK and showed that a nurse led catch up at school resulted in more MMR vaccinations than a reminder to have a catch-up vaccination at a general practice. The committee used this study to provide support for their recommendation for school-based catch-up sessions. However, this study only looked at MMR vaccinations and was a cohort study rather than an RCT, so the results were judged to be of low quality. Some evidence in the qualitative review evaluated the use of a catch-up campaign for young people, based in GP surgeries (see evidence review B). Based on the importance of enabling children and young people who have missed vaccinations to be identified and vaccinated later, the committee agreed that despite the findings of the studies above more research was still needed on this topic. They therefore made a research recommendation to compare the effectiveness and acceptability of school-based and GP-based catch-up campaigns in the UK (see Appendix K). This will help providers establish the most appropriate and acceptable setting or settings for catch-up sessions as these may vary depending on the vaccination.
The committee also discussed the importance of tailoring interventions to increase vaccine uptake to specific communities to increase their effectiveness. They were aware that the WHO has a specific programme called ‘Tailoring Immunisation Programmes’ (TIP) but noted that this approach had not been evaluated to determine whether it was effective as a method of designing and testing interventions to increase routine vaccine. The committee therefore wrote a research recommendation on this topic (see Appendix K for more details).
Consent for non-school-based vaccinations
The committee noted that vaccinations that are given in schools differ from other vaccines, as this is the only scenario where the person giving consent is not the person receiving the vaccine, and they are not with the person receiving the vaccine at the time of vaccination as is the case with parents of young children. Given these differences in the vaccination process, the committee did not think that the evidence for incentivised consent form return could be applied to other vaccination groups, such as babies and children, pregnant women or people who are aged 65 years and over. Instead, it was decided that additional evidence is needed to identify whether incentives are also effective at increasing vaccination uptake in these other groups. This supported the findings of the infrastructure review that more research is needed to determine the most effective methods to increase vaccine uptake for all ages and groups. The committee therefore made a research recommendation aimed at examining whether incentives are also effective at increasing vaccine uptake in these groups (see Appendix K, evidence review G).
Although most of the evidence relating to consent in this review was for young people aged 11–18 years, the committee discussed how issues relating to vaccine consent can also be a barrier to uptake for some groups of adults. This is particularly important for individuals who need support with decision making or who may lack the mental capacity to consent for vaccination and are at risk of not being vaccinated as a result. Although there was no evidence for these other populations, the committee thought it was important to promote equality by ensuring that all people are given the support necessary to make informed decisions on vaccination. An additional recommendation was therefore included which links to the NICE guideline on decision making and mental capacity. This will provide clinicians with guidance on what to consider when discussing consent for non-school-based vaccinations. The committee were also aware that there is a NICE guideline on Advocacy services for adults with health and social care needs that is being developed and is likely to be relevant to this topic when it publishes in 2022.
Future proofing the recommendations
In the evidence reviews we looked for evidence regarding routine vaccinations for people aged 65 and over because this was the age limit for vaccinations for older people on the NHS routine schedule at the time the work was carried out. Since there was limited evidence for this age group, we also included data from relevant studies including people aged 50 and over, where the majority of participants were in our target age group, or the mean age was 65 or over with committee agreement taken on a review-by-review basis. These studies were downgraded for applicability where the committee deemed it appropriate.
According to the Joint Committee on Vaccination and Immunisation minutes from the meeting on 22 June 2021 shingles vaccination eligibility is changing to include people aged 60 and over and this will be introduced in a phased manner down from the current age of 70 years. It is unclear when this change will be initiated or completed. In order to future proof the guideline recommendations we have therefore changed those mentioning people aged 65 and over to refer to older people instead and defined them as follows: adults who are eligible for routine vaccination on the UK schedule, excluding pregnancy-related vaccinations. We also suggest that people consult the green book for information about current age limits and vaccinations for older people. The content of the recommendations has not been changed otherwise as this was not deemed necessary. The majority of recommendations that apply to older people are also more generally applicable and have not been altered because they do not mention groups of people by age. The committee discussions of the evidence have also been retained in their original form, with the addition of the information about the use of the term older people where the relevant recommendations that specifically mentioned people aged 65 and over are discussed.
1.1.12. Recommendations supported by this evidence review
This evidence review supports recommendations 1.2.6, 1.3.6, 1.3.28, 1.3.27, 1.3.30, 1.3.34–1.3.37 and the research recommendations on incentives for school-based vaccinations; GP versus school-based catch-up campaigns and the use of the World Health Organisation ‘Tailoring Immunisation Programmes’ (TIP) approach in designing interventions to increase vaccine uptake. Other evidence supporting these recommendations can be found in: evidence review B: the barriers to and facilitators to vaccine uptake; evidence review, evidence review D: increasing vaccine uptake by improving access; E: education interventions to increase the uptake of routine vaccines.
1.1.13. References – included studies
- Chantler T, Pringle E, Bell S et al (2020) Does electronic consent improve the logistics and uptake of HPV vaccination in adolescent girls? A mixed-methods theory informed evaluation of a pilot intervention. BMJ open 10(11): e038963 [PMC free article: PMC7640514] [PubMed: 33148741]
- Audrey S, Evans K, Farr M, Ferrie J, Yates J, Roderick M FH (2021) Implementing new consent procedures for schools-based human papillomavirus vaccination: a qualitative study. British Journal of Child Health 2(2)
- Audrey, S., Farr, M., Roderick, M. et al (2020) How acceptable is adolescent self-consent for the HPV vaccination: Findings from a qualitative study in south-west England. Vaccine 38(47): 7472–7478 [PMC free article: PMC7604563] [PubMed: 33041101]
- Fisher, H., Evans, K., Ferrie, J. et al (2020) Young women's autonomy and information needs in the schools-based HPV vaccination programme: a qualitative study. BMC public health 20(1): 1680 [PMC free article: PMC7654043] [PubMed: 33172415]
- Jackson, C., Cheater, F.M., Peacock, R. et al (2010) Evaluating a web-based MMR decision aid to support informed decision-making by UK parents: A before-and-after feasibility study. Health Education Journal 69(1): 74–83
- Lwembe S, Green SA, Tanna N et al A qualitative evaluation to explore the suitability, feasibility and acceptability of using a 'celebration card' intervention in primary care to improve the uptake of childhood vaccinations. BMC family practice 17: 101 [PMC free article: PMC4967527] [PubMed: 27475527]
- Rockliffe L; Stearns S; Forster AS (2020) A qualitative exploration of using financial incentives to improve vaccination uptake via consent form return in female adolescents in London. PloS one 15(8): e0237805 [PMC free article: PMC7446903] [PubMed: 32822387]
- Rockliffe, Lauren, Chorley, Amanda J, McBride, Emily et al (2018) Assessing the acceptability of incentivising HPV vaccination consent form return as a means of increasing uptake. BMC public health 18(1): 382 [PMC free article: PMC5859432] [PubMed: 29558923]
- Fisher H, Hickman M, Ferrie J et al (2020) Impact of new consent procedures on uptake of the schools-based human papillomavirus (HPV) vaccination programme. Journal of public health (Oxford, England) [PMC free article: PMC8904199] [PubMed: 32978614]
- Forster, Alice S, Cornelius, Victoria, Rockliffe, Lauren et al (2017) A cluster randomised feasibility study of an adolescent incentive intervention to increase uptake of HPV vaccination. British journal of cancer 117(8): 1121–1127 [PMC free article: PMC5674104] [PubMed: 28829766]
- Gibson K (2014) Celebrate and Protect: A mixed methods evaluation.: https://static1
.squarespace .com/static/50b4ab77e4b0214dc1f631e9 /t/542acb2ce4b0664ced44ffea /1412090668767 /Celebrate+and+Protect+Evaluation+Report+Final+October+2014.pdf [Accessed 26/11/2020] - Shourie, S; Jackson, C; Cheater, F M; Bekker, H L; Edlin, R; Tubeuf, S; Harrison, W; McAleese, E; Schweiger, M; Bleasby, B; Hammond, L; A cluster randomised controlled trial of a web based decision aid to support parents' decisions about their child's Measles Mumps and Rubella (MMR) vaccination.; Vaccine; 2013; vol. 31 (no. 50); 6003–10 [PMC free article: PMC3898271] [PubMed: 24148574]
- Altinoluk-Davis F; Gray S; Bray I; Measuring the effectiveness of catch-up MMR delivered by school nurses compared to signposting to general practice on improving MMR coverage.; Journal of public health (Oxford, England); vol. 42 (no. 2) [PubMed: 32052033]
- Chantler, T; Letley, L; Paterson, P et al (2019) Optimising informed consent in school-based adolescent vaccination programmes in England: A multiple methods analysis. Vaccine 37: 5218–5224. [PubMed: 31351797]
- Letley, L., Rew, V., Ahmed, R., et al (2018) Tailoring immunisation programmes: Using behavioural insights to identify barriers and enablers to childhood immunisations in a Jewish community in London, UK. Vaccine 36(31):4687–4692. [PubMed: 29945834]
- Seok J; Heffernan C; Mounier-Jack S; Chantler T; Perspectives of vaccinators on the factors affecting uptake of meningococcal ACWY vaccine amongst school leavers in London.; Public health; 2018; vol. 164. [PubMed: 30292165]
Mixed methods
Qualitative
Quantitative
Other
Appendices
Appendix A. Review protocol
Review protocol of the barriers to, and facilitators for, vaccine uptake and interventions to increase vaccine uptake.
Please note that the review protocol includes a quantitative question about interventions to increase uptake. This part of the work is presented in evidence review C to ensure the size of the evidence reviews remains manageable.
Download PDF (325K)
Appendix B. Literature search strategies
A search for qualitative evidence to answer the review question what are the barriers to, and facilitators for, increasing the uptake of routine vaccines? was run on 31st December 2019 and 10th January 2020 in the following databases Medline, Medline in Process, Medline Epub ahead of print, Embase, Emcare and Psycinfo (all via the Ovid Platform), the Cochrane Database of Systematic Reviews (via the Wiley 2015 Platform), Applied Social Sciences Index and Abstracts, Sociological Abstracts and British Nursing Index (all via the Proquest platform). The Medline strategy is shown below. NICE inhouse qualitative and OECD country geographic filters were used where appropriate and the search limited to records published since 1990 and in the English language. The strategy was translated for all databases and re run on ddmmyy.
Download PDF (126K)
Appendix C. Qualitative evidence study selection
Download PDF (185K)
Appendix D. Evidence tables
Mixed methods evidence
Download PDF (180K)
Qualitative evidence
Download PDF (420K)
Quantitative evidence
Download PDF (263K)
Appendix E. Forest plots and table of results
Vaccination of babies and children aged 0–5 years (Celebrate and Protect Programme)
Table 17. Results from the Celebrate and Protect Programme (cluster non-randomised controlled trial (PDF, 116K)
MMR vaccine uptake in babies and children aged 0–5 years using a decision aid (cluster RCT- data adjusted for clustering)
Download PDF (115K)
HPV vaccine uptake in young people aged 11–18 years using electronic consent forms (cluster non-randomised controlled trial)
Download PDF (115K)
HPV vaccination consent form return in young people aged 11–18 years using incentivised consent form return (cluster RCT- data adjusted for clustering)
Download PDF (118K)
HPV vaccine uptake in young people aged 11–18 years using a new process to obtain consent on vaccination day (controlled before-after study)
Download PDF (118K)
Appendix F. GRADE-CERQual and GRADE tables
F.1. GRADE-CerQual tables
F.1.1. Babies and children aged 0–5 years
F.1.2. Young people aged 11–18 years
Table 20. Themes for the vaccination of young people aged 11–18 years using electronic consent forms (PDF, 131K)
Table 21. Themes for the vaccination of young people aged 11–18 years using incentivised consent forms (PDF, 140K)
F.2. GRADE tables
F.2.1. Babies and children aged 0–5 years
Table 23. Babies and children aged 0–5 years using the Celebrate and Protect (reminders) programme1 (PDF, 128K)
Table 24. Babies and children aged 0–5 years using an MMR web-based decision aid (PDF, 111K)
F.2.2. Young people aged 11–18 years
Table 25. Young people aged 11–18 years using electronic consent forms (PDF, 125K)
Table 26. Young people aged 11–18 years using incentivised consent forms (PDF, 113K)
Table 27. Young people aged 11–18 years using a new process to obtain consent on vaccination day (PDF, 114K)
Appendix G. Economic evidence study selection
None of the economic evidence identified was relevant for this review question.
Download PDF (145K)
Appendix H. Economic evidence tables
No economic evidence was identified for this review question.
Appendix I. Health economic model
The committee discussed incentives for consent form return for school-based vaccinations and, due to the anticipated resource impact, a costing analysis was undertaken to better estimate the costs associated with this intervention.
The costing exercise was based on the Forster 2017 study. In this study the incentive for consent form return was a 1 in 10 chance of winning a £50 voucher. For the costing analysis this incentive scenario was used as the base-case, and alternative values for incentives were selected by the committee based on things they felt could be implementable in practice, and used as additional scenarios. These alternative values were; one person per school chance of winning a £50 voucher, a fixed amount of £3 per student, and a free in-school perk such as a lunch queue pass. In each case, the effectiveness data from the Forster study were used, as it is not known how differences in the incentive used would affect changes in consent form return. The Forster study reported numbers of positive consent forms returned, which was taken as a proxy for vaccination, as the committee considered it unlikely many children with positive consent forms would fail to be vaccinated.
The incentive scheme for consent form return would be implemented before vaccinations are scheduled to be given, and before any other uptake interventions can be applied, compared to the same scenario but without this initial incentive scheme. It is expected that using an incentive scheme would increase the numbers of consent forms returned, and therefore reduce the resource use in follow-up of people who did not return forms. The analysis therefore compares two strategies; one where an incentive is offered and then reminders given to those still not returning consent forms, and a second option where no incentive is offered, and then reminders are given to those not returning consent forms
The cost per person receiving the incentive was calculated for each scenario in Table 28. Data on the number of pupils and number of schools in England was taken from government education statistics (GOV.UK; Schools, pupils and their characteristics 2020/2021), with 615,634 pupils in school year 8 and 3,456 secondary schools in England.
Table 28. Costs of incentive scenarios (PDF, 108K)
The committee discussion indicated that in usual practice if consent forms have not been returned, a nurse will phone the individual/parent/carer to obtain consent. The cost of this follow-up phone call was assumed to be £7.80 per call based on the PSSRU estimate for a telephone appointment lasting 6.56 minutes with a practice nurse. For costing purposes, a practice nurse was assumed to be a reasonable proxy for school nurses who would likely be those making the phone calls.
The uptake data was taken from Forster 2017 for the incentives intervention and from Ferson 1995 and Vivier 2000 for the phone reminder, and are presented in Table 29.
Table 29. Uptake data (PDF, 120K)
Vaccine uptake with phone reminders only
To calculate the change in uptake using phone reminders only (Table 30), the baseline uptake probability of 88.01% is first adjusted using the control arm uptake data from the phone reminders studies to account for those who would be vaccinated without the phone call, giving a probability of vaccine uptake before phone call reminder of 84.06%. This adjustment is necessary because phone call reminders are used in current practice in some areas, so it is likely that the real-world baseline uptake data includes people who have had a phone call reminder. This adjustment is designed to estimate the proportion of people who would be vaccinated before the impact of any reminder or incentive intervention is included. The baseline odds of uptake before phone reminders in the relevant RCTs was 0.33 (calculated from the probability of 24.79%) and applying the OR of 2.34 gives an odds of uptake with phone reminders of 0.77 or a probability of 43.54%. Applying this additional 43.54% to those not already vaccinated gives a total probability of vaccination with phone call reminders of 91%.
Table 30. Calculation of uptake with phone reminders only (PDF, 109K)
Vaccine uptake with incentives plus phone reminder
The baseline uptake rate for the HPV vaccine is 88.01% (GOV.UK; HPV vaccination coverage in adolescent females in England 2018–2019), giving a baseline odds of being vaccinated of 7.34. Applying the OR of 2.06, the odds of positive consent form return with the incentive intervention is calculated to be 15.09, or a probability of 93.79%. To add in the phone call reminders component this 93.79% is adjusted using the control arm data for the reminder intervention, where the control arm uptake rate was 24.79%, giving a post-incentive pre-reminder uptake of 91.74%. Applying the 43.54% for additional uptake with phone reminders, the probability of vaccine uptake after the incentive and phone reminder is 95.33%.
Table 31. Calculation of uptake with incentives and phone reminders (PDF, 113K)
The cost of the incentive plus phone reminder combination is calculated as the incentive cost per person plus the cost of the phone reminder only applied to those who did not return positive consent forms following the incentive, i.e. 100% – 93.79% = 6.21%. The incremental cost per additional person vaccinated (using positive consent form return as a proxy for vaccination) for the incentive plus phone reminder combination compared with phone reminders only was calculated by taking the difference in costs over the difference in uptake and is presented alongside the cost per person for the incentive combination in Table 32. In Table 32 and Table 33 two of the incentive scenarios are dominant and have a negative ICER compared with reminder only, which means these interventions are less costly than reminders only and result in higher overall uptake.
Table 32. Cost-effectiveness of incentives in the average baseline uptake scenario (PDF, 112K)
A low uptake scenario was also considered, using a baseline uptake of 70.2%, from the local authority with the lowest HPV uptake reported in 2018/19. The results of the low uptake scenario are presented in Table 33, and the incentive plus reminder combination is more cost-effective in this scenario, with an ICER of £34.07 in the base-case. This is primarily because in areas with lower uptake, the successful use of incentives to increase consent form return rates reduces the amount of follow-up nurses have to do with families who have not returned forms.
Table 33. Cost-effectiveness of incentives in the low baseline uptake scenario (PDF, 99K)
Appendix J. Excluded studies
Clinical studies
Excluded from the original search
Download PDF (893K)
Excluded from the re-runs search
Download PDF (232K)
Economic studies
Download PDF (143K)
Appendix K. Research recommendations – full details
K.1.1. Research recommendation 1
What levels and types of incentives are effective and acceptable for increasing vaccination uptake in a school-based population in the UK?
K.1.2. Why this is important
One of the barriers to vaccine uptake for school-based populations is the non-return of consent forms. There is evidence from a UK study (Forster 2017) to indicate that offering financial incentives to young people for the return of consent forms can increase the number of consent forms that are returned, and that the majority of these returned forms contained consent for the vaccination, although there was no direct data on uptake. It is unclear whether other types of incentives (either financial or non-financial) would be effective in a school-based population; what levels of incentives are required to be effective and whether incentivising uptake directly is an effective approach.
K.1.3. Rationale for research recommendation
Download PDF (138K)
K.1.4. Modified PICO table
Download PDF (115K)
K.1.5. Research recommendation 2
Is the use of the World Health Organisation ‘Tailoring Immunisation Programmes’ (TIP) approach an effective way of designing interventions to increase vaccine uptake in a UK context?
K.1.6. Why this is important
The WHO TIP approach aims to understand the barriers to vaccination among population groups with suboptimal coverage and then facilitate the design of interventions to overcome these barriers thereby increasing vaccine uptake. In the reviews for this guideline limited evidence was identified that used this approach and it is unclear whether this is an effective method of developing tailored vaccination programmes aimed at increasing vaccination uptake. However, this approach has been used in the UK (for example, PHE has a document on Tailoring Immunisation Programmes in the Charedi community in North London and this is published as Letley 2018). It may therefore be the case that this evidence exists but was not identified as part of the current work because:
- of the format used to report it (if it is not a peer-reviewed publication)
- the published qualitative work does not match the format of the included studies for the qualitative review (i.e., focus groups, interviews or open-ended questionnaire questions) and present the barriers and enablers in a more summarised format than could be incorporated in our synthesis of the qualitative findings.
- the studies do not report the effect on vaccine uptake (the key outcome for the quantitative reviews).
- we did not record whether studies that used the TIP approach to design their interventions because this was not part of the review protocol.
If the TIP approach is effective at increasing uptake of routine vaccinations in the UK then it could be a used by providers of routine vaccination programmes to develop strategies tailored their local communities. Research is therefore needed to identify whether literature already exists to facilitate examination of the effectiveness of this approach at increasing vaccine uptake. If no such literature exists, then research aimed at using the TIP approach to design an intervention (or a multi-component intervention) and determine the effects on routine vaccine uptake would be needed to address this research recommendation.
K.1.7. Rationale for research recommendation
Download PDF (151K)
K.1.8. Modified PICO table
Download PDF (142K)
K.1.9. Research recommendation 3
What is the effectiveness and acceptability of school-based catch-up vaccination sessions compared with GP-based catch-up campaigns in the UK?
K.1.10. Why this is important
The reviews for this guideline identified limited evidence about the effectiveness of different types of catch-up campaigns for routine vaccinations, and only one study (Altinoluk-Davis 2020) considered the effectiveness of catch-up campaigns delivered in schools compared to GP surgeries for MMR vaccination. An effective catch-up campaign can help to increase the number of people who are up to date with their routine vaccinations by giving people who have missed vaccinations another opportunity to be vaccinated. (Catch-up campaigns in response to disease outbreaks were out of scope and not included in the evidence reviews.) For children and young people in the UK these catch-up sessions routinely occur within schools, but vaccinations may also be offered at GPs. It is unclear whether school-based or GP-based catch-up campaigns are more effective for these age groups for vaccinations other than MMR and information about what makes an effective and acceptable campaign could be used to improve their design and implementation.
K.1.11. Rationale for research recommendation
Download PDF (154K)
K.1.12. Modified PICO table
Download PDF (145K)
Final version
Evidence review underpinning recommendations 1.2.6, 1.3.6, 1.3.28, 1.3.27, 1.3.30, 1.3.34 to 1.3.37 and 3 research recommendations in the NICE guideline
This evidence review was developed by the Guideline Development Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Evidence review for the barriers to, and facilitators for, vaccine uptake: Vaccine uptake in the general population: Evidence review B[ 2022]Review Evidence review for the barriers to, and facilitators for, vaccine uptake: Vaccine uptake in the general population: Evidence review B. 2022 May
- Review Evidence review for identification and recording of vaccination eligibility and status: Vaccine uptake in the general population: Evidence review A[ 2022]Review Evidence review for identification and recording of vaccination eligibility and status: Vaccine uptake in the general population: Evidence review A. 2022 May
- Review Evidence review for multicomponent interventions to increase the uptake of routine vaccines: Vaccine uptake in the general population: Evidence review H[ 2022]Review Evidence review for multicomponent interventions to increase the uptake of routine vaccines: Vaccine uptake in the general population: Evidence review H. 2022 May
- Review Evidence review for interventions to increase the uptake of routine vaccines by improving infrastructure: Vaccine uptake in the general population: Evidence review G[ 2022]Review Evidence review for interventions to increase the uptake of routine vaccines by improving infrastructure: Vaccine uptake in the general population: Evidence review G. 2022 May
- Review Interventions to increase vaccine uptake by targeting acceptability: Vaccine uptake in the general population: Evidence review I[ 2022]Review Interventions to increase vaccine uptake by targeting acceptability: Vaccine uptake in the general population: Evidence review I. 2022 May
- Evidence review for the acceptability and effectiveness of interventions to incr...Evidence review for the acceptability and effectiveness of interventions to increase routine vaccine uptake
Your browsing activity is empty.
Activity recording is turned off.
See more...
